Abstract
Specific to the West African sub-region, previous studies involving fruit, stem, and bark of Tetrapleura tetraptera as well as seeds of Monodora myristica have largely focused on phytochemical properties of aqueous and methanolic and ethanolic extracts. To supplement existing information, the chemical composition, antibacterial efficacy (tested against Escherichia coli and Staphylococcus aureus), and antioxidant capacity (1,1-diphenyl-2-picrylhydrazyl (DPPH∙) radical scavenging, ferric reducing power, and total antioxidant capacity) of essential oil and oleoresin extracted from T. tetraptera fruit and M. myristica seeds cultivated in Southeast Nigeria, were studied. Essential oil and oleoresin were respectively extracted by steam distillation and aqueous maceration. By way of gas chromatograph mass spectrometry (GC–MS) analysis, the chemical compounds from essential oil and oleoresin from M. myristica and T. Tetraptera samples totaled 6 and 5, as well as 27 and 16, respectively. Besides the oleoresin of M. myristica and the essential oil of T. tetraptera showing some resistance against S. aureus, the oleoresins seemed highly susceptible to E. coli—all of which demonstrated concentration-dependence to the antibacterial inhibition zone. Scavenging DPPH radical, reduction power activity, and total antioxidant capacity increased with essential oil and oleoresin extracts' concentrations, which positions M. myristica and T. tetraptera spices as very promising for food preservation, especially against autoxidation and microbial spoilage.
Similar content being viewed by others
Introduction
Globally, edible plants continue to be of research interest given their natural compounds and medicinal properties that are capable of improving health and preventing/combating diseases1. Spices are among such particularly large groups of edible plants with beneficial natural ingredients usually added to foods. Furthermore, the capacity to influence both aroma and taste of foods is largely owed to the presence of essential/volatile oils that comprise terpenes and terpenoids along with various aliphatic hydrocarbons, acids, alcohols, etc.2, able to control food spoilage and prolong shelf life3. In addition to essential oils, spices show potential health benefits through antimicrobial, antioxidant, antidiabetic, anti-inflammatory, anti-viral, and antiprotozoal capacities. Additionally, the chemical composition and medicinal properties could be influenced by genetics and the type of extraction method3. In addition to their abundance from rural to urban areas, spices from African flora have helped in the discovery of novel drugs useful in biomedicine, which have helped to promote health and tackle diseases such as cancer, tumors, etc. Examples of indigenous spices in Africa include black pepper (Xylopia aethiopica), West African pepper (Piper guineese), Mentha piperita, Ocimum gratissimum, Tetrapleura tetraptera and Monodora myristica. Unlike the exotic types, most of the above-named spices are wholly used and continue to receive research attention given the reported phytochemical constituents, antioxidant, and antimicrobial capacities1,3,4,5,6,7.
On one hand, the T. tetraptera is a deciduous perennial flowering medicinal plant of West African origin belonging to the pea family(Fabaceae), and commonly known as aridan and yanayan amongst the Yoruba and Urhobos respectively in Nigeria and prekese in Ghana4,5. The essential oil present in T. tetraptera provide a distinctive pleasant fragrance and aroma, making its dry fruit a popular seasoning spice in South-eastern Nigeria5. Ghanaians believe that T. tetraptera fruits contain multivitamins, useful in the management of jaundice, inflammation, convulsion, fever, and leprosy1,4,8. The phytochemical constituents of T. tetraptera fruits provide medicinal attributes demonstrated by molluscicidal, antimicrobial, antioxidant, anticonvulsant, anti-inflammatory, antimalarial, antidiabetic, and anticancer properties8,9,10,11. Microbiological studies show the extract of T. tetraptera exhibits excellent antimicrobial activity against Gram-positive and Gram-negative bacteria4,12. On the other hand, the M. myristica, also known as African/calabash nutmeg, is a perennial plant of the family Annonaceae, which thrives in the tropics of West Africa and the Caribbean. Common names of this plant in Nigeria include ehuru, ariwo, ehiri, airama, and awerewa, lubushi6. The seed is rich in oil and is of great value due to its medicinal and nutritional qualities3. The fruits and seeds are used as stimulants, stomachic, against headaches, and sores, and as a natural insect repellent. The essential oil contains compounds like α-phellandrene, α-pinene, myrcene, limonene, and pinene. The pleasant aroma, like nutmeg, makes this spice useful in the preparation of traditional dishes in Nigerian communities6. Spice seed extracts are believed to possess both antioxidant1,7 and antimicrobial properties3. M. myristica could be effective in the treatment of stomach aches, febrile pains, eye diseases, and hemorrhoids13, while Tetrapleura tetraptera helps to tackle diabetes mellitus, arthritis, hypertension, epilepsy, and asthma5.
Both T. tetraptera and M. myristica are among such common indigenous spices in Nigeria that are under-utilized/-valued, despite their sharp aroma and flavor that is highly perceivable by the sense of smell3,4. However, specific to West Africa sub-region, previous studies involving fruit, stem, and bark of T. tetraptera as well as seeds of M. myristica have largely focused on phytochemical properties of aqueous and methanolic and ethanolic extracts. There is a need, therefore, for further investigations into the essential oil and oleoresin of this T. tetraptera and M. myristica, specifically their chemical composition, antioxidant, and antibacterial properties and such studies would require respective activities of steam distillation maceration technique, which would help unravel their potential/relevance in the various communities in southeast Nigeria where they serve as a natural preservative to prevent food spoilage. To supplement existing information, this current work investigated the chemical composition, antibacterial efficacy, and antioxidant capacity of essential oil and oleoresin extracted from T. tetraptera fruit and M. myristica seeds cultivated in Southeast Nigeria. Specifically, the essential oil and oleoresin respectively extracted by steam distillation and aqueous maceration were subsequently subject to analytical tests in adherence to the relevant institutional guidelines. Furthering the knowledge and understanding underpinning the capacities of these extracted essential oil and oleoresin to tackle food spoilage challenges is warranted to help consolidate the product development potential of both T. tetraptera fruit and M. myristica seeds.
Materials and methods
Schematic overview of the experimental program
Figure 1 shows the schematic overview of the experimental program of this current study, which depicts the major stages from procurement of plant materials, and processing of plant parts extraction processes, before the subsequent analytical measurements. For emphasis, this conducted research was directed to understand how the extracted essential oil and oleoresin from T. tetraptera fruit and M. myristica seeds would thrive specific to the context of chemical composition, antibacterial efficacy (tested against Escherichia coli and Staphylococcus aureus), and antioxidant capacity (1,1-diphenyl-2-picrylhydrazyl (DPPH∙) radical scavenging, ferric reducing power (FRAP), and total antioxidant capacity (TAC). Additionally, the analytical measurements were carried out independently using the different essential oil and oleoresin samples obtained from individual batches of T. tetraptera and M. myristica seeds. Importantly, the analytical measurements conducted were in adherence to the relevant guidelines prescribed by the Department of Food Science and Technology, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria. Furthermore, all chemicals and reagents used in this work, which were procured from reputable registered chemical retailers, were of analytical grade standard.
Procurement and processing of plant materials
Fresh T. tetraptera fruit (approx. 1000 g) and M. myristica seeds (approx. 800 g) were procured as two separate batches from a local market in Owerri Imo State, in the Southeast of Nigeria. Furthermore, Fig. 2 shows the photo images of M. myristica seeds (Fig. 2a) and T. tetraptera fruits (Fig. 2b). To further confirm the samples, the samples were identified at the Department of Plant Biotechnology of Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria. The processing of fresh T. tetraptera fruit and M. myristica seeds was carried out following the method described by14 with modifications. Specifically, the spices were washed with clean running water, sun-dried (72 h), and then oven dried using a Memmert UN30 (Memmert GmbH + Co. KG, Schwabach, Germany) oven at 55 °C for 72 h. The M. myristica seeds were cracked manually to recover the nibs, whereas the fruits of T. tetraptera were cut into small pieces, and both were kept in ambient conditions until required for further processing.
Extraction processes of essential oil and oleoresin
Already cracked M. myristica seeds, and small pieced cut T. tetraptera fruit samples were individually ground into coarse and fine particles using an attrition type mill K-Tron type T-35volumetric feeder (K-Tron Corp. Pitman NJ USA). The coarse particles were kept for essential oil extraction, whereas those of fine powder was for oleoresin extraction. On one hand, essential oil extraction involved the steam distillation method as described by Chemat et al.15 with slight modification. Briefly, the conventional type steam distillation apparatus with a cylindrical Pyrex body supported by Teflon grid at its lower end, with a rheostat-controlled heating facility, was used. The essential oil was dried over anhydrous sodium sulfate (Na2SO4) and stored in airtight amber bottles at 4 °C until used. On the other hand, the oleoresin extraction involved the maceration technique modified from Fernández‐Ronco et al.16. For each spice, 200 g of finely ground spice was macerated in 400 ml of distilled water in a 1000 ml conical flask and tightly corked. This was shaken vigorously at 30-min intervals for 3 h, after which the supernatant was decanted and the residue was filtered out. After extraction, the supernatant from each spice was collected and evaporated at 60 °C in a stifling air oven (Memmert UN30, Memmert GmbH + Co. KG, Schwabach, Germany) for 8 h. The oleoresins were stored in airtight amber bottles and kept in the refrigerator until used.
Analytical measurements
Determination of chemical composition
The chemical composition of the crude essential oil and oleoresin samples was determined following the routine method employed in the laboratory of the Department of Food Science and Technology, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria. This involved the gas chromatograph mass spectrometry (GC–MS) analysis, specifically the Agilent 6890 gas chromatograph (Supelco, Bellefonte, PA, USA) equipped with an on-column automatic injector, flame ionization detector, and HP 88 capillary column (100 m × 0.25 μm film thickness). The carrier gas was helium at a constant flow rate of 1.0 mL/min. The detector was maintained at 250 °C and both injectors at 220 °C with integrator chart speed at 2 cm/min. The initial column temperature was set at 100 °C (hold time = ~ 2 min) to the final temperature of 180 °C at a rate of 50 °C/min, the volume injected was 1.0 μL and the split ratio was 50:1. Total chromatogram was auto-integrated by Shem-Station, and emergent chemical constituents were identified by comparison with both published mass spectral database (NIST02.L) and available literature data.
Determination of antibacterial efficacy
Measurement of inhibition zone
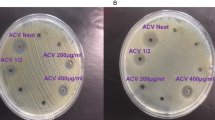
The agar well diffusion method according to Balouiri, Sadiki, and Ibnsouda17 was used. The microorganisms (0.1 mL) from previously prepared diluents were spread on previously prepared nutrient agar in standard Petri dishes in triplicates. Wells of 2 mL diameter and 0.5 mL depth were created using a sterile bore. The essential oil and oleoresin samples were introduced using a sterile micropipette, after which Petri dishes were stored at 37 °C for 24 h in an incubator to observe and measure the zone of inhibition (mm) of microbial growth caused by the aforementioned substances.
Monitoring of pathogen growth and survival
Staphylococcus aureus ATCC 27840 and Escherichia coli 0157, routinely used for the antibacterial assay, were obtained from the microbiology department of the University of Nigeria Nsukka, Nigeria. Substrates were refrigerated at 4 °C for 5 days and the growth and survival of the pathogens were monitored using the Plate colony-counting method. Using 1.0 mL from each pathogen Escherichia coli, and Staphylococcus aureus, suspended to a density of 105, were inoculated into a flask with nutrient agar. Extracts diluted in 0.1% buffered peptone water to appropriate concentrations, subsequently mixed thoroughly, and aseptically poured onto a petri dish. Colonies were manually counted periodically within 5 days of incubated storage at 37 °C.
Determination of antioxidant capacities
Free radical scavenging activity
Free radical scavenging activity of the already extracted essential oil and oleoresin were measured using the radical 1, 1-diphenyl-2-Picrylhydrazyl (DPPH) as described by Shah et al.18 with slight modifications. This involved dissolving ~ 25 mg of DPPH in 100 mL methanol, and thereafter storing at 20 °C till needed. From the working solution obtained from the DPPH stock solution by methanol, 1 mL was added to 100 μL of various concentrations of test samples, incubated for 45 min, thoroughly shaken, and then left in the dark under ambient conditions. Ascorbic acid served as the standard, scavenging effect was calculated by Eq. (1):
ADPPH = absorbance of DPPH at 517 nm and AEOIL = absorbance of oils/resins at 517 nm.
Iron (III) to iron (II)-reducing power assay (FRAP)
The ability of essential oil and oleoresin to reduce Fe3+ to Fe2+ was assessed by the method of Hinneburg, Dorman, and Hiltunen19 with slight modifications. A 2-mL aliquot of sample was added to 2.5 mL of sodium phosphate buffer (0.2 M, pH 6.6, 2.5 mL) containing potassium ferricyanide [K3Fe (CN)6] (1%, 2.5 mL). After the reaction mixture had incubated at 50 °C for 25 min, then 2.5 mL of trichloroacetic acid (10%) was added and centrifuged at 3000 rpm for 10 min. Subsequently, the supernatant (5 mL) was mixed with 2.5 mL of water, and 0.5 mL of 0.1% aqueous FeCl3, followed by an absorbance reading at 700 nm. Gallic acid was used as a standard reference compound.
Phosphomolybdate assay (total antioxidant capacity)
The total antioxidant capacity (TAC) of the essential oil and oleoresin was determined by the phosphomolybdate method of Jan et al.20 with slight modifications. An aliquot (~ 0.1-mL) of the sample was mixed with 1 mL reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate), thereafter covered and incubated in a water bath at 85 °C for 75 min. When samples had been cooled, the absorbance was measured at 765 nm. The ascorbic acid was used as a standard reference compound. The TAC was estimated using the Eq. (2) below:
Statistical analysis
The data of three replicates from different samples were subjected to factorial approach analysis of variance (ANOVA). Results were represented as means ± standard error, with mean separation conducted using Fisher’s least significant difference (LSD) per measured variable. The probability level was statistically significant at p < 0.05 (95% confidence). Minitab 2018 software (Minitab, LLC, USA) was used to run the data.
Results and discussion
Chemical composition of essential oil and oleoresin
The main chemical compounds of essential oil and oleoresin of M. myristica and T. tetraptera are shown in Tables 1 and 2, with their respective GC–MS chromatograms shown in Figs. 3, 4, 5 and 6. Specific to M. myristica, the identified chemical compounds predominantly monoterpenes respectively totalled 6 and 5 in essential oil and oleoresin. Chemical compounds in essential oil would include p-cymene at 50.58% as peak, followed by α-phellandrene at 32.09%, with α-pinene at 8.71% as least, whereas in oleoresin would include, 2-acetyl cyclopentanone (85.42%) as predominant, with α-phellandrene (6.40%) as least. Only δ-cadinene (1.56%) appeared in the sesquiterpene but very little. Previous reports about different extracts of M. myristica specific to Nigeria obtained variations in chemical constituents. For example, a report of seed oil of M. myristica from southwest Nigeria obtained major constituents like α-phellandrene epoxide (3.02%), carvacrol (2.09%), and δ-cadinene (2,21%)21, as well as p-cymene (31.5%), α-phellandrene (18.1%), α-pinene (6.1%), β-pinene (5.1%)22. Elsewhere also, essential oil of M. myristica obtained by steam distillation showed chemical constituents like α-phellandrene (50.4%), α-pinene (5.5%), myrcene (4.35%), and germacrene-d-4-ol (9.0%)23, whereas another seed oil reported elsewhere showed chemical constituents like germacrene-d-4-ol (25.48%), tricyclo [5.2.1(1,5)] dec-2-ene (13.35%), δ-cadinene (11.09%) and linalool (15.10%), and from their stem barks with chemical constituents like γ-cadinene (31.31%), α-elemene (17.98)13. In a neighbouring country of Chad and Cameroon, the essential oils extracted by hydrodistillation from fruits of Xylopia aethiopica (Dunal) A. Rich, Xylopia parviflora (A. Rich) Benth.) and M. myristica (Gaertn) showed α-phellandrene (Chad = 52.7%; Cameroon = 67.1%) as a major constituent24. Specific to T. Tetraptera, the chemical compounds of essential oil and oleoresin respectively totalled 27 and 16 (Tables 2 and 3, as well as Figs. 2, 3, 4 and 5). On one hand, the essential oil comprised carboxylic acids, phenolic acids, and esters, for example, 1-naphthalenecarbonitrile, 2-methoxy- (8.26%), dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester (7.90%), benzenamine, 4-methoxy-N-(triphenylphosphoranylidene)- (7.78%), 4-dibenzofuranamine (7.08), etc. On the other hand, the oleoresin comprised monoterpenes, for example, γ-terpinene (25.63%), linalool (20.74%), caryophyllene (9.68%), β-pinene (9.38%), 2-carene (6.98), γ-elemene (5.47%), etc. Terpenes were not found in the essential oil of T. tetraptera. The essential oil extracted from T. tetraptera fruits via hydro-distillation by Erukainure et al.4 largely showed acetic and carboxylic acid, with little traces of terpenes. Probably, the presence of linalool might account for the plant’s peppery nature, whereas both γ-terpinene and α-thujene might account for the flavor/fragrance. Elsewhere, the root and stem ethanol extract of T. tetraptera spice respectively obtained 70.14%25 and 32.01%10. Polyphenols like eugenol, quercetin, rutin, and tyrosol, according to Moukette et al.1, were considered 5.88–10.79-fold more concentrated in the water and ethanolic extracts of the fruits compared to the barks. Furthermore, α-phellandrene and α-pinene has been identified as a major chemotype for M. myristica seeds, whereas citral and linalool as major chemotypes of T. tetraptera1,4,21,22. Such factors as environmental influences, experimental conditions, place of origin, post-harvest handling of the fruits, adaptive metabolism of plants, and plant part analyzed might be responsible for the variations in the chemical compounds that have been detected in M. myristica and T. tetraptera samples at this current work.
Antibacterial efficacy of essential oil and oleoresin
As mentioned earlier in this paper, there is evidence that the extract of T. tetraptera would exhibit very promising antimicrobial activity against Gram-positive/-negative bacteria4,12. Table 3 shows the antibacterial inhibition zone of essential oils and oleoresins of M. myristica and T. tetraptera, which appeared concentration-dependent to the bacterial pathogens. Whereas oleoresin obtained the highest susceptibility to E. coli, those of M. myristica together with the essential oil of T. tetraptera showed some resistance to S aureus. Considering that the inhibition zone qualitatively deciphers the potentials of an antibacterial agent, the essential oil of M. myristica at concentration of 75 µg/mL obtained the highest zone (16 mm) against E. coli whereas the essential oil from T. tetraptera obtained the least activity. Elsewhere, inhibition zones of 8 mm have been reported and 13 mm for 2.5 mg/mL and 5.0 mg/mL, respectively, of essential oil M. myristica against E. coli3, whereas 11 mm for 2 mL also of M. myristica essential oil against E. coli26. Another study showed T. tetraptera stem extract against two Salmonella strains, with a remarkably high inhibition zone (> 20 mm diameter)10, besides the absence of carvone component believed as responsible for the weak activity of oleoresins27,28. Comparatively, the promising antibacterial activity of essential oil from M. myristica might be dependent on factors like chemical composition and its solubility3. The extraction process, cell wall composition of microbial entity, environmental conditions, chemotype of the plant parts used (which often influences biosynthetic pathways), as well as other physiological properties might also potential factors that contribute to the differences in the antibacterial activity.
Natural compounds (terpenes inclusive), which are secondary metabolites from plants, are responsible for the defense against pathogens29 and promising in controlling pathogenic/spoilage microbial entities in foods. The survival of the E. coli and S. aureus population involving different concentrations of Mondora myristica and Tetrapleura tetraptera essential oil and oleoresin can be seen in Table 4. Of both spices, the survival of E. coli and S. aureus population appeared significantly higher (p < 0.05) in the essential oil above those of oleoresin. Within three (3) days of storage, specific to concentrations 25 and 50 µg/mL of essential oil from T. tetraptera, the pathogen population increased markedly (p < 0.05) at 2.15 × 105 and 1.53 × 105 CFU/mL (E. coli) and 2.5 and 2.03 × 105 CFU/mL (S. aureus), respectively. Within 3 days of storage, however, no bacteria proliferated in T. tetraptera oleoresin as well as M. myristica essential oil and oleoresin. By day 5, the T. tetraptera essential oil respectively showed 7.05 and 6.10 × 105 CFU/mL for E. coli and S. aureus (Table 5). However, S. aureus obtained 6.51 and 4 × 107 CFU/mL at 25 and 50 µg/mL for M. myristica essential oil, significantly different(p < 0.05) from 1.5 × 104 CFU/mL at 25 µg/mL for T. tetraptera oleoresin. Gram-positive bacteria are believed to reveal more susceptibility to antibacterial compounds compared to Gram-negative bacteria28,30, given the lipoproteins and lipopolysaccharides present in the cellular wall/barrier-like structure that restricts the entry of hydrophobic compounds31. In essential oil, however, presence of terpenes should provide the major antibacterial capacity. More so, some simple phenols might appear in the form of phenylpropanoids32. Besides essential oils and oleoresins of this current work being concentration-dependent, their complex mix of several (minor) chemical components would assert multifunctional synergistic effects33,34.
Antioxidant capacity of essential oil and oleoresin
The DPPH radical scavenging activity of essential oils and oleoresins (%) at different concentrations in M. myristica and T. tetraptera spices can be seen in Table 5. In general, the extracts showed promising scavenging activity with a maximum of 93.46% and a minimum of 53.76%. DPPH radical scavenging activity increased with concentration except for T. tetraptera essential oil. The oleoresin of T. tetraptera showed the better DPPH radical scavenging activity across samples’ concentrations. For example, the highest concentration (640 µg/mL) of T. tetraptera oleoresin obtained maximum scavenging power (93.46%) that appeared significantly higher (p < 0.05) compared to the others, which specifically demonstrates enhanced antioxidant properties. Previous reports show roughly 29–37.7% scavenging powers of M. myristica seeds could concur with maximum concentration of 120 µg/mL35, contrasting 10–41% when concentration is maximum at 400 µg/mL7. Lower DPPH scavenging has been recorded at T. tetraptera fruit and bark ethanolic and hydroethanolic extracts1,4,36. Typically through the reduction of DPPH, the radical scavenging compounds (OH, O2−, L., LOO., LO.,) act like an antioxidant during lipid oxidation, given the ability to donate an electron or hydrogen molecule37. Of all reactive oxygen species, the OH radical appears the stronger, and able to react with most biological molecules found in living cells. Among effective defences of living body against various diseases is the removal of hydroxyl radicals38.
The reducing power activity (%) of essential oils and oleoresins at different concentrations in M. myristica and T. tetraptera spices can be seen in Table 6. Like the DPPH radical scavenging, the reducing power activity would increase with concentration, and more evident at the essential oil of M. myristica of this study. Specifically, M. myristica essential oil obtained the peak highest reducing power with mean absorbance value of 54.69 at concentration of 640 µg/mL. Despite the higher values at concentrations higher than 100 µg/mL7, the ethanol extract of T. tetraptera fruit peel (at similar concentrations higher than 100 µg/mL) might possess the lesser reducing power4, although the higher reducing power for ethanolic and hydroethanolic extracts of fruit and bark could resemble1. Acting as a defense mechanism against lipid peroxidation, the antioxidants in plant materials would bind to metal ions by reducing transition metals such as Fe2+ or Cu+33. Further, the reducing power of essential oils and oleoresins take place largely because the reductones can transfer electrons or hydrogen atoms that break the free radical chain4. The total antioxidant capacity (TAC) of essential oils and oleoresins (%) at different concentrations in M. myristica and T. tetraptera spices can be seen in Table 7. Like the DPPH radical scavenging and reducing power activity, the TAC would also increase with concentration, being more evident at the oleoresin of M. myristica of this study. The TAC of both essential oils and oleoresins can be said to be dose dependent. M. myristica essential oil obtained the peak TAC value (15.75) at 640 µg/mL concentration, significantly higher (p < 0.05) than that of 360 µg/mL (TAC value = 8.76). T. tetraptera oleoresin obtained the second-highest absorbance values, while M. myristica oleoresins had the least absorbance levels. The TAC recorded in this current work seemed higher than those of M. myristica35, including those of aqueous and ethanolic extracts of fruits, pulps, and seeds of T. tetraptera8. Nonetheless and for emphasis, the TAC assay is based on the ability of a reducing agent (antioxidant) to reduce molybdenum (VI) ions to molybdenum (V) giving a green phosphomolybdate (V) complex at 765 nm20,37,39.
Conclusions
In this study and from M. myristica and T. tetraptera spices found in the Southeast of Nigeria, both essential oil and oleoresin were secured, respectively, by steam distillation and maceration. Very useful compounds were obtained, through which some considerable antibacterial and antioxidant potentials were exhibited. Essential oil of M. myristica appeared to have enhanced antibacterial and antioxidant activity compared to the others. The T. tetraptera, however, seemed less effective to inhibit both E. coli and S. aureus populations. The extraction process, environment, and genetics would be crucial influences on the natural compounds found in each spice’s essential oil and oleoresin, which would in turn strongly impact (both M. myristica and T. tetraptera spices’) antibacterial and antioxidant capabilities, and hold great promise in food preservation, especially against the autoxidation and microbial aspects of (food) spoilage. Considering the findings of this current work, the direction of future investigations should be to establish the purity of these chemical constituents of essential oil and oleoresin obtained from M. myristica and T. tetraptera spices, and their corresponding cytotoxic activities, which could possibly help better the understanding that underpin their pharmacological and other associated/related effects.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Moukette, B. M. et al. In vitro ion chelating, antioxidative mechanism of extracts from fruits and barks of Tetrapleura tetraptera and their protective effects against fenton mediated toxicity of metal ions on liver homogenates. Evid.-based Complement. Altern. Med. 2015, 423689. https://doi.org/10.1155/2015/423689 (2015).
Omar, J. et al. Quantitative analysis of bioactive compounds from aromatic plants by means of dynamic headspace extraction and multiple headspace extraction-gas chromatography-mass spectrometry: quantitative analysis of bioactive compound. J. Food Sci. 81, C867–C873. https://doi.org/10.1111/1750-3841.13257 (2016).
David, O. M., Ojo, O. O., Olumekun, V. O. & Famurewa, O. Antimicrobial activities of essential oils from Hura crepitans (L.), Monodora myristica (Gaert Dunal) and Xylopia aethiopica (Dunal A. Rich) seeds. Br. J. Appl. Sci. Technol. 4, 3332–3341 (2014).
Erukainure, O. L. et al. Ethanol extract of Tetrapleura tetraptera fruit peels: Chemical characterization, and antioxidant potentials against free radicals and lipid peroxidation in hepatic tissues. J. Taibah Univ. Sci. 11, 861–867. https://doi.org/10.1016/j.jtusci.2017.03.007 (2017).
Udourioh, G. A. & Etokudoh, M. F. Essential oils and fatty acids composition of dry fruits of Tetrapleura tetraptera. J. Appl. SCI. Environ. Manag. 18, 419–424 (2014).
Ezeuko, A. S., Bamgboye, O. A., Jonathan, H. & Uchenna, D. Extraction, physicochemical, phytochemical analysis, and identification of some important compounds of Monodora myristica (African nutmeg) seed oil. IJIRAS. 4, 406–410 (2017).
Akinwunmi, K. F. & Oyedapo, O. O. Evaluation of antioxidant potentials of Monodora myristica (Gaertn) dunel seeds. Afr. J. Food Sci. 7, 317–324 (2015).
Adusei, S., Otchere, J. K., Oteng, P., Mensah, R. Q. & Tei-Mensah, E. Phytochemical analysis, antioxidant, and metal chelating capacity of Tetrapleura tetraptera. Heliyon 5, e02762. https://doi.org/10.1016/j.heliyon.2019.e02762 (2019).
Ogbunugafor, H. A., Ugochukwu, C. G. & Kyrian-Ogbonna, A. E. The role of spices in nutrition and health: A review of three popular spices used in Southern Nigeria. Food Qual. Saf. 1, 171–185. https://doi.org/10.1093/fqsafe/fyx020 (2017).
Agyemang, K. et al. Antibacterial activity, and mechanism of Tetrapleura tetraptera stem extract against Salmonella strains and its application in raw chicken meat. J. Food Process. Preserv. 45, e14489 (2021).
Mbaveng, A. T. et al. Cytotoxic phytochemicals from the crude extract of Tetrapleura tetraptera fruits towards multi-factorial drug resistant cancer cells. J. Ethnopharmacol. 267, 113632. https://doi.org/10.1016/j.jep.2020.113632 (2021).
Ekwenye, U. N. & Okorie, C. F. Antibacterial activity of Tetrapleura tetraptera Taub.pod extracts. Int. J. Pharm. Biol. Sci. 1, 731–741 (2010).
Owokotamo, I. & Ekundayo, O. Comparative study of the essential oils of Monodora myristica from Nigeria. Eur. Chem. Bull. 1, 263–265. https://doi.org/10.17628/ECB.2012.1.263 (2012).
Abayeh, O. J., Abdulrazaq, A. K. & Olaogun, R. Quality characteristics of Canarium schweinfurthii Engl. oil. Plant Foods Hum. Nutr. 54, 43–48 (1999).
Chemat, F. et al. Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean, and environmentally friendly approach. Anal. Chim. Acta 555, 157–160 (2006).
Fernández-Ronco, M. P., Gracia, I., de Lucas, A. & Rodríguez, J. F. Extraction of Capsicum annuum oleoresin by maceration and ultrasound-assisted extraction: Influence of parameters and process modeling. J. Food Process Eng. 36, 343–352 (2013).
Balouiri, M., Sadiki, M. & Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 6, 71–79. https://doi.org/10.1016/j.jpha.2015.11.005 (2016).
Shah, N. A. et al. Investigation on flavonoid composition and anti-free radical potential of Sida cordata. BMC Complement. Altern. Med. 13, 276 (2013).
Hinneburg, I., Dorman, H. J. D. & Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 97, 122–129 (2006).
Jan, S., Khan, M. R., Rashid, U. & Bokhari, J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca Buxifolia fruit. Osong. Public Health Res. Perspect. 4, 246–254 (2013).
Igwe, C. C., Yayi, E. & Moudachirou, M. Chemical constituents of the solvents extracted and hydrodistilled essential oils of African nutmeg (Monodora myristica) and turmeric (Cucrcuma domestica) in Southwest Nigeria. Niger. Food J. 23, 21–32 (2005).
Owolabi, M. S. et al. Bioactivity of three plant derived essential oils against the maize weevils Sitophilus zeamais (Motschulsky) and cowpea weevils Callosobruchus maculatus (Fabricius). Elec. J. Environ. Agricult. Food Chem. 8, 828–835 (2009).
Onyenekwe, P. C., Ogbadu, G., Deslaurier, H., Gagnon, M. & Collin, G. J. Volatile constituents of the essentisal oils of Mondora myristica (Gaertn) dunal. J. Sci. Food Agric. 61, 379–381 (1993).
Bakarnga-Via, I. et al. Composition and cytotoxic activity of essential oils from Xylopia aethiopica (Dunal) A. Rich, Xylopia parviflora (A. Rich) Benth.) and Monodora myristica (Gaertn) growing in Chad and Cameroon. BMC Complement. Altern. Med. 14, 1–8. https://doi.org/10.1186/1472-6882-14-125 (2014).
Lin, L., Agyemang, K., Abdel-Samie, M. A. & Cui, H. Antibacterial mechanism of Tetrapleura tetraptera extract against Escherichia coli and Staphylococcus aureus and its application in pork. J. Food Saf. 39, e12693 (2019).
Adewole, E. et al. Phytochemical, antimicrobial, and Gc-Ms of African nutmeg (Monodora Myristica). Int. J. Pharm. Sci. Invent. 2, 25–32 (2013).
Martino, L. D., Feo, V. D., Formisano, C., Mignola, E. & Senatore, F. Chemical composition & antimicrobial activity of the essential oils from three chemotypes of origanum vulgare l.ssp. hirtum (link) Ietswaart growing wild in Campania (Southern Italy). Molecules 14, 2735–2746 (2009).
Singh, S. et al. Comparative studies of chemical composition, antioxidant and antimicrobial potentials of essential oils and oleoresins obtained from seeds and leaves of Anethum graveolens L. Toxicol. Open Access 3, 119. https://doi.org/10.4172/2476-2067.1000119 (2017).
Pichersky, E. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity". Science 311, 808–811. https://doi.org/10.1126/science.1118510 (2006).
Burt, S. Essential oils: Their antibacterial properties and potential applications in foods: A review. Int. J. Food Microbiol. 94, 223–253 (2004).
Cox, S. D. & Markham, J. L. Susceptibility, and intrinsic tolerance of Pseudomonas aeruginosa to selected plant volatile compounds. J. Appl. Microbiol. 103, 930–936 (2007).
Aldred, E. Phenols. In Pharmacology: A Handbook for Complementary Healthcare Professionals (eds Aldred, E. et al.) 149–166 (Churchill Livingstone, 2009).
Koroch, A. R., Rodolfo Juliani, H. & Zygadlo, J. A. Bioactivity of essential oils and their components. In Flavours and Fragrances (ed. Berger, R. G.) 87–115 (Springer, 2007).
Singh, S. et al. Comparison of chemical composition, antioxidant and antimicrobial potentials of essential oils and oleoresins obtained from seeds of brassica juncea and sinapis Alba. MOJ Food Process. Technol. (MOJFPT) 4, 113–120. https://doi.org/10.15406/mojfpt.2017.04.00100 (2017).
Uyoh, E. A., Chukwurah, P., Akarika, C. R. & Antia, V. A. Potentials of two Nigerian spices—Piper nigrum and Monodora myristica as sources for cheap natural antioxidants. Am. J. Plant Sci. 4, 1105–1115. https://doi.org/10.4236/ajps.2013.45137 (2013).
Famobuwa, O., Lajide, L., Owolabi, B., Osho, I. & Amuho, U. Antioxidant activity of the fruit and stem bark of Tetrapleura tetraptera Taub (Mimosaceae). Br. J. Pharm. Res. 9, 1–4. https://doi.org/10.9734/bjpr/2016/21462 (2016).
Ahmed, D., Khan, M. M. & Saeed, R. Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antioxidants 4, 394–409. https://doi.org/10.3390/antiox4020394 (2015).
Jae-Young, J., Pyo-jam, P. & Se-kwon, K. Antioxidant activity of a peptide isolated from Alaska Pollack (Theragra chalcogramma) frames protein hydrolysate. Food Res. Int. 38(1), 45–50. https://doi.org/10.1016/j.foodres.2004.07.005 (2005).
Mashwani, Z., Khan, M.A., Irum, S. & Ahmad, M. Antioxidant potential of root bark of Berberis lycium Royle. from Galliyat, western Himalaya, Pakistan. Pak. J. Bot. 231–234. (2013)
Acknowledgements
Author Q.N.O. gratefully acknowledges the research funding from the Ministry of Science and Higher Education of the Russian Federation (Ural Federal University Program of Development within the Priority-2030 Program). Author S.J. and C.O.R.O. appreciate the financial support from Wrocław University of Environmental and Life Sciences, Poland.
Author information
Authors and Affiliations
Contributions
Q.N.O., and F.U.U., were associated with conceptualization; data curation; formal analysis; investigation; methodology; and wrote the original draft; C.E.O. was associated with data curation; formal analysis; methodology; and reviewed and edited the final manuscript; S.J. was associated with funding acquisition, visualization, methodology, reviewed and edited the final manuscript; C.O.R.O. was associated with methodology, funding acquisition, project administration, validation, visualization, reviewed and edited the final manuscript. All authors agree to the last version submitted for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okechukwu, Q.N., Ugwuona, F.U., Ofoedu, C.E. et al. Chemical composition, antibacterial efficacy, and antioxidant capacity of essential oil and oleoresin from Monodora myristica and Tetrapleura tetraptera in Southeast Nigeria. Sci Rep 12, 19861 (2022). https://doi.org/10.1038/s41598-022-23161-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23161-5
This article is cited by
-
Chemical characterization, safety profile and antileiomyoma effects of Tetrapleura tetraptera Taubert (Fabaceae) fruit ethanol extract in Sprague Dawley rats
Future Journal of Pharmaceutical Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.