A New Benzopyranyl Cadenane Sesquiterpene and Other Antiplasmodial and Cytotoxic Metabolites from Cleistochlamys kirkii
Abstract
:1. Introduction
2. Results and Discussion
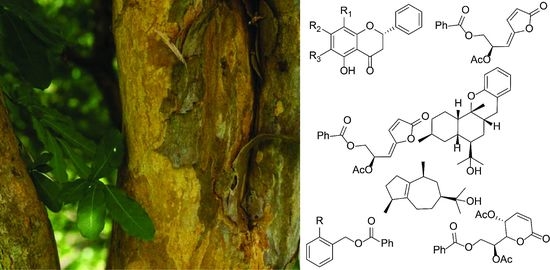
2.1. Isolation and Identification of Compounds
2.2. Antiplasmodial, Cytotoxic and Translation Inhibition Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation of Compounds
3.4. Antiplasmodial Asexual Assay
3.5. Cytotoxicity Assay
3.6. Translation Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verdcourt, B. Flora of tropical East Africa: Annonaceae; Royal Botanic Gardens, Kew: London, UK, 1971; Volume 5, p. 131. [Google Scholar]
- Nyandoro, S.S.; Munissi, J.J.; Gruhonjic, A.; Duffy, S.; Pan, F.; Puttreddy, R.; Holleran, J.P.; Fitzpatrick, P.A.; Pelletier, J.; Avery, V.M.; et al. Polyoxygenated cyclohexenes and other constituents of Cleistochlamys kirkii leaves. J. Nat. Prod. 2017, 80, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Samwel, S.; Mdachi, S.J.M.; Nkunya, M.H.H.; Irungu, B.N.; Moshi, M.J.; Moulton, B.; Luisi, B.S. Cleistenolide and cleistodienol: Novel bioactive constituents of Cleistochlamys kirkii. Nat. Prod. Commun. 2007, 2, 737–741. [Google Scholar] [CrossRef]
- Nyandoro, S.S. Some rare Tanzanian plant species as sources of less common metabolites: Biomedical potential and conservation status. J. Pharmacogn. Phytochem. 2014, 3, 147–157. [Google Scholar]
- Verzár, R.; Petri, G. Medicinal plants in Mozambique and their popular use. J. Ethnopharm. 1987, 19, 67–80. [Google Scholar] [CrossRef]
- Bordoloi, M.; Shukla, V.S.; Nath, S.C.; Sharma, R.P. Naturally occurring cadinenes. Phytochemistry 1989, 28, 2007–2037. [Google Scholar] [CrossRef]
- Chyu, C.F.; Ke, M.R.; Chang, Y.S.; Chien, S.C.; Kuo, Y.H. New Cadinane-type sesquiterpenes from the roots of Taiwania Cryptomerioides Hayata. Helv. Chim. Acta. 2007, 90, 1514–1521. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, C.H.; Chien, S.C.; Lin, Y.L. Five new cadinane-type sesquiterpenes from the heartwood of Chamaecyparis obtusa var. formosana. J. Nat. Prod. 2002, 65, 25–28. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chyu, C.F.; Lin, H.C. Cadinane-type sesquiterpenes from the roots of Taiwania cryptomerioides Hayata. Chem. Pharm. Bull. 2003, 51, 986–989. [Google Scholar] [CrossRef]
- Muhammad, I.; Waterman, P.G. Chemistry of the Annonaceae, Part XXVI. The Uvarisesquiterpenes, a novel type of benzylated sesquiterpene from Uvaria angolensis. J. Nat. Prod. 1988, 51, 719–724. [Google Scholar] [CrossRef]
- Mgani, Q. Uvarisesquiterpene D: A new benzopyranyl sesquiterpene isolated from Uvaria Lucida ssp. Lucida. Tanzan. J. Sci. 2012, 38, 65–71. [Google Scholar]
- Weenen, H.; Nkunya, M.H.; El-Fadl, A.A.; Harkema, S.; Zwanenburg, B. Lucidene, a bis (benzopyranyl) sesquiterpene from Uvaria lucida ssp. lucida. J. Org. Chem. 1990, 55, 5107–5109. [Google Scholar] [CrossRef]
- Weenen, H.; Nkunya, M.H.; Mgani, Q.A.; Posthumus, M.A.; Waibel, R.; Achenbach, H. Tanzanene, a spiro benzopyranyl sesquiterpene from Uvaria tanzaniae Verdc. J. Org. Chem. 1991, 56, 5865–5867. [Google Scholar] [CrossRef]
- Achenbach, H.; Höhn, M.; Waibel, R.; Nkunya, M.H.; Jonker, S.A.; Muhie, S. Oxygenated pyrenes, their potential biosynthetic precursor and benzylated dihydroflavones from two African Uvaria species. Phytochemistry 1997, 44, 359–364. [Google Scholar] [CrossRef]
- Pereira, F.; Madureira, A.M.; Sancha, S.; Mulhovo, S.; Luo, X.; Duarte, A.; Ferreira, M.J.U. Cleistochlamys kirkii chemical constituents: Antibacterial activity and synergistic effects against resistant Staphylococcus aureus strains. J. Ethnopharmacol. 2016, 178, 180–187. [Google Scholar] [CrossRef]
- Lasswell, W.L., Jr.; Hufford, C.D. Cytotoxic C-benzylated flavonoids from Uvaria chamae. J. Org. Chem. 1977, 42, 1295–1302. [Google Scholar] [CrossRef]
- Ching, A.Y.L.; Wah, T.S.; Sukari, M.A.; Lian, G.E.C.; Rahmani, M.; Khalid, K. Characterization of flavonoid derivatives from Boesenbergia rotunda (L.). Malays. J. Anal. Sci. 2007, 11, 154–159. [Google Scholar]
- Kodpinid, M.; Thebtaranonth, C.; Thebtaranonth, Y. Benzyl benzoates and o-hydroxybenzyl flavanones from Uvaria ferruginea. Phytochemistry 1985, 24, 3071–3072. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.J.; Xie, H.Q.; Mu, Q. Guaiol—A naturally occurring insecticidal sesquiterpene. Nat. Prod. Commun. 2013, 8, 1353–1354. [Google Scholar] [CrossRef]
- Jung, J.; Pummangura, S.; Chaichantipyuth, C.; Patarapanich, C.; Fanwick, P.; Chang, C.J.; McLaughlin, J. New bioactive heptenes from Melodorum fruticosum (Annonaceae). Tetrahedron 1990, 46, 5043–5054. [Google Scholar] [CrossRef]
- Slade, D.; Ferreira, D.; Marais, J.P.J. Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry 2005, 66, 2177–2215. [Google Scholar] [CrossRef]
- Antus, S.; Baitzgacs, E.; Kajtar, J.; Snatzke, G.; Tokes, A.L. Circular-Dichroism and Absolute-Configuration of Azaflavanones and Thiaflavanones. Liebigs. Ann. Chem. 1994, 497–502. [Google Scholar] [CrossRef]
- Atilaw, Y.; Duffy, S.; Heydenreich, M.; Muiva-Mutisya, L.; Avery, V.M.; Erdelyi, M.; Yenesew, A. Three chalconoids and a pterocarpene from the roots of Tephrosia aequilata. Molecules 2017, 22, 318. [Google Scholar] [CrossRef]
- Deyou, T.; Gumula, I.; Pang, F.; Gruhonjic, A.; Mumo, M.; Holleran, J.; Duffy, S.; Fitzpatrick, P.A.; Heydenreich, M.; Landberg, G.; et al. Rotenoids, flavonoids, and chalcones from the root bark of Millettia usaramensis. J. Nat. Prod. 2015, 78, 2932–2939. [Google Scholar] [CrossRef]
- Nkunya, M.H.H. Natural Chemicals for Disease and Insect Management; Professorial Inaugural Lecture; University of Dar es Salaam: Dar es Salaam, Tanzania, 2002. [Google Scholar]
- Duffy, S.; Avery, V.M. Development and optimization of a novel 384-well anti-malarial imaging assay validated for high-throughput screening. Am. J. Trop. Med. Hyg. 2012, 86, 84–92. [Google Scholar] [CrossRef]
- Novac, O.; Guenier, A.S.; Pelletier, J. Inhibitors of protein synthesis identified by a high throughput multiplexed translation screen. Nucleic. Acids. Res. 2004, 32, 902–915. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds 1–13 are available from the authors. |




| Position | δH (m, J, Hz) | δC |
|---|---|---|
| 1 | 2.50 (ddd, 10.7, 7.7, 2.1) | 43.6 |
| 2α | 1.81 (dddd, 12.8, 11.0, 10.9, 7.1) | 26.5 |
| 2β | 1.59 (m) | |
| 3α | 1.50 (dddd, 11,9, 11.6, 5.8, 2.0) | 32.8 |
| 3β | 1.35 (12.3, 7.3, 2.6, 2.6) | |
| 4 | 2.08 (m) | 39.0 |
| 5α | 1.75 (ddd, 13.7, 3.6, 1.7) | 29.0 |
| 5β | 1.15 (ddd, 13.7, 10.2, 3.0) | |
| 6 | 2.13 (dddd, 12.9, 10.7, 6.7, 3.8) | 43.3 |
| 7 | 1.54 (m) | 46.4 |
| 8α | 1.91 (ddd, 14.3, 4.8, 2.4) | 31.6 |
| 8β | 1.64 (ddd, 14.3, 11.2, 3.8) | |
| 9 | 2.27 (dddd, 12.3, 4.9, 4.6, 4.6) | 40.3 |
| 10 | - | 81.4 |
| 11 | - | 73.9 |
| 12 | 1.22 (s) | 27.6 |
| 13 | 1.19 (s) | 26.6 |
| 14 | 1.47 (s) | 24.6 |
| 15 | 0.86 (d, 7.2) | 16.0 |
| 1′ | - | 153.5 |
| 2′ | 6.75 (d, 8.0) | 117.3 |
| 3′ | 7.07 (dd, 8.0, 8.0) | 127.3 |
| 4′ | 6.81(dd,8.0, 8.0) | 119.7 |
| 5′ | 7.04 (d, 8.0) | 129.3 |
| 6′ | - | 123.0 |
| 7′α | 2.91 (dd, 16.4, 12.3) | 28.4 |
| 7′β | 2.54 (dd, 16.4, 4.9) |
| Compound/Extract | 3D7 | MDA-MB-231 |
|---|---|---|
| CKRE (crude) | 72% c.* | 42.0 a (μg/mL) |
| Cleistonol (1) | IA b | NT |
| Chamanetin (2) | 62% b | 22.7 a |
| Isochamanetin (3) | 72% b | 11.6 a |
| Dichamanetin (4) | 9.3 a | 9.6 a |
| 7-Methoxyisochamanetin (5) | 84% b | NT |
| Pinostrobin (6) | IA b | NT |
| Pinocembrin (7) | 10% b | 30.7 a |
| Benzylbenzoate (8) | 25% b | NT |
| 2-Methoxybenzylbenzoate (9) | IA b | NT |
| Guaiol (10) | IA b | NT |
| Polycarpol (11) | 6% b | NT |
| (E)-Acetylmelodorinol (12) | 7.6 a | 13.2 a |
| Cleistenolide (13) | 15.2 a | 18.6 a |
| Lupeol (reference) | 0.089 | |
| Artesunate (reference) | 0.00048 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyandoro, S.S.; Maeda, G.; Munissi, J.J.E.; Gruhonjic, A.; Fitzpatrick, P.A.; Lindblad, S.; Duffy, S.; Pelletier, J.; Pan, F.; Puttreddy, R.; et al. A New Benzopyranyl Cadenane Sesquiterpene and Other Antiplasmodial and Cytotoxic Metabolites from Cleistochlamys kirkii. Molecules 2019, 24, 2746. https://doi.org/10.3390/molecules24152746
Nyandoro SS, Maeda G, Munissi JJE, Gruhonjic A, Fitzpatrick PA, Lindblad S, Duffy S, Pelletier J, Pan F, Puttreddy R, et al. A New Benzopyranyl Cadenane Sesquiterpene and Other Antiplasmodial and Cytotoxic Metabolites from Cleistochlamys kirkii. Molecules. 2019; 24(15):2746. https://doi.org/10.3390/molecules24152746
Chicago/Turabian StyleNyandoro, Stephen S., Gasper Maeda, Joan J.E. Munissi, Amra Gruhonjic, Paul A. Fitzpatrick, Sofia Lindblad, Sandra Duffy, Jerry Pelletier, Fangfang Pan, Rakesh Puttreddy, and et al. 2019. "A New Benzopyranyl Cadenane Sesquiterpene and Other Antiplasmodial and Cytotoxic Metabolites from Cleistochlamys kirkii" Molecules 24, no. 15: 2746. https://doi.org/10.3390/molecules24152746