Chrysopetalinae, Ehlers, 1864
|
publication ID |
https://doi.org/ 10.1080/00222933.2017.1395919 |
|
persistent identifier |
https://treatment.plazi.org/id/03E91002-8728-135F-FF0B-FE82FB88FA55 |
|
treatment provided by |
Felipe |
|
scientific name |
Chrysopetalinae |
| status |
|
Subfamily Chrysopetalinae View in CoL
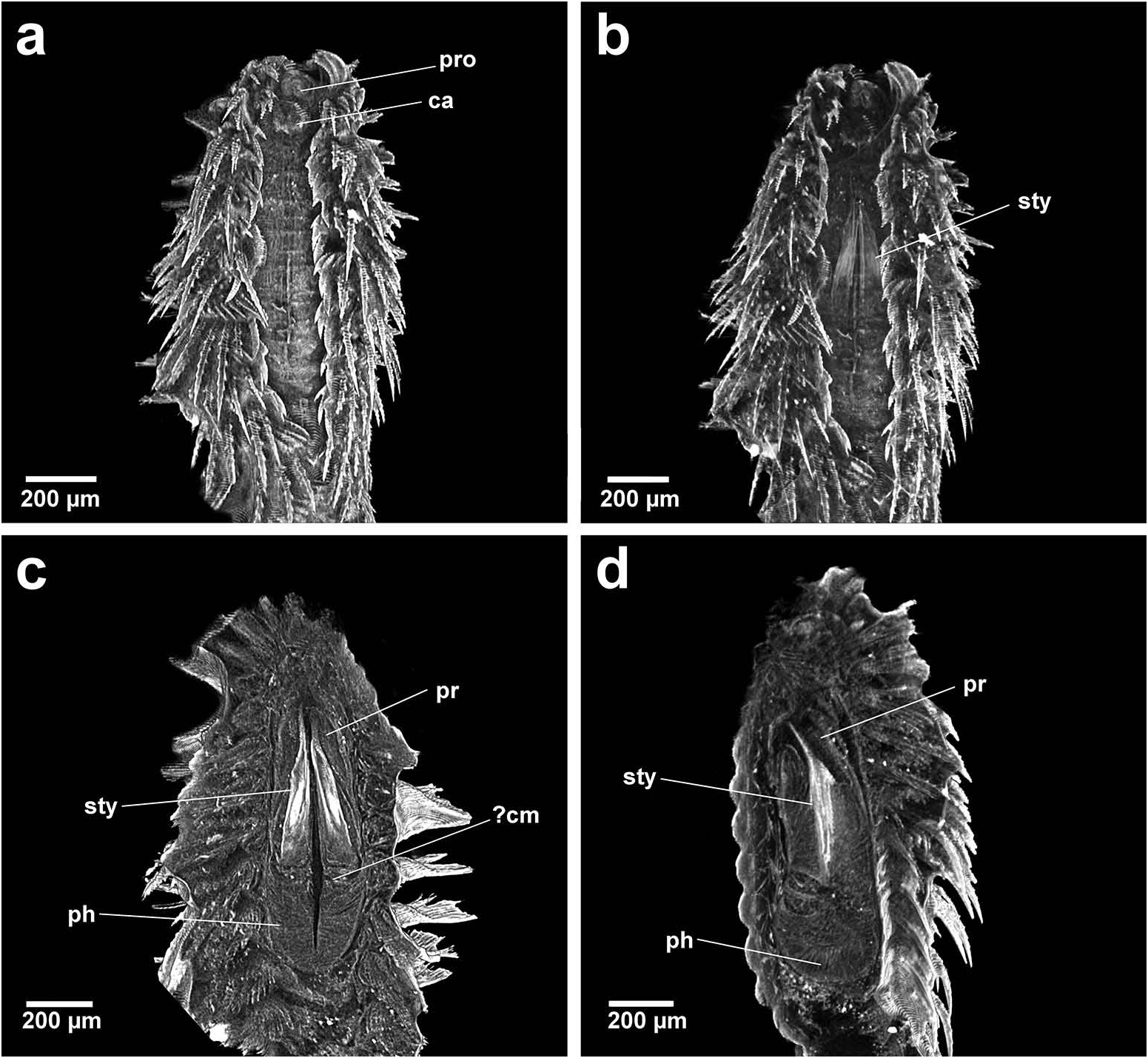
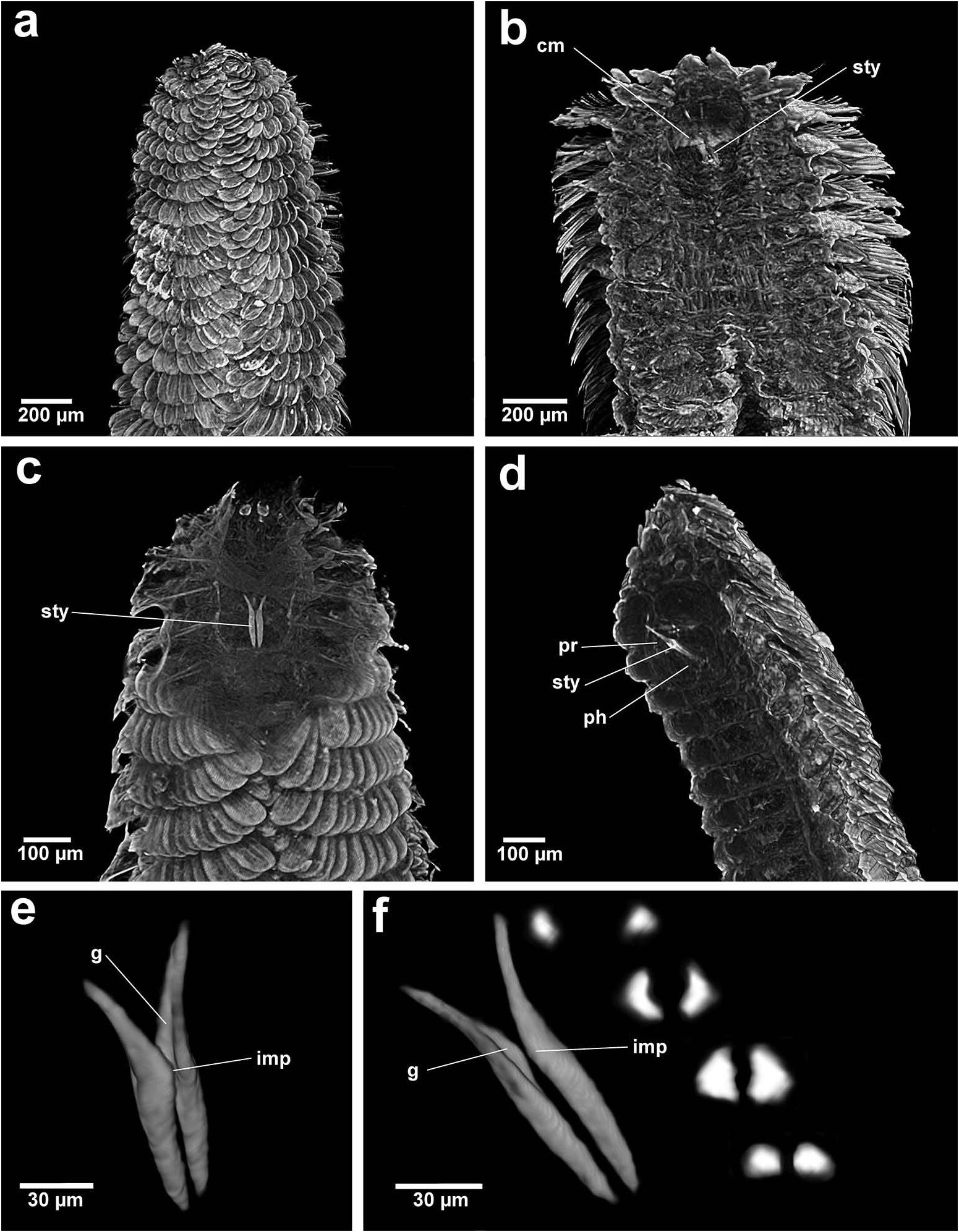
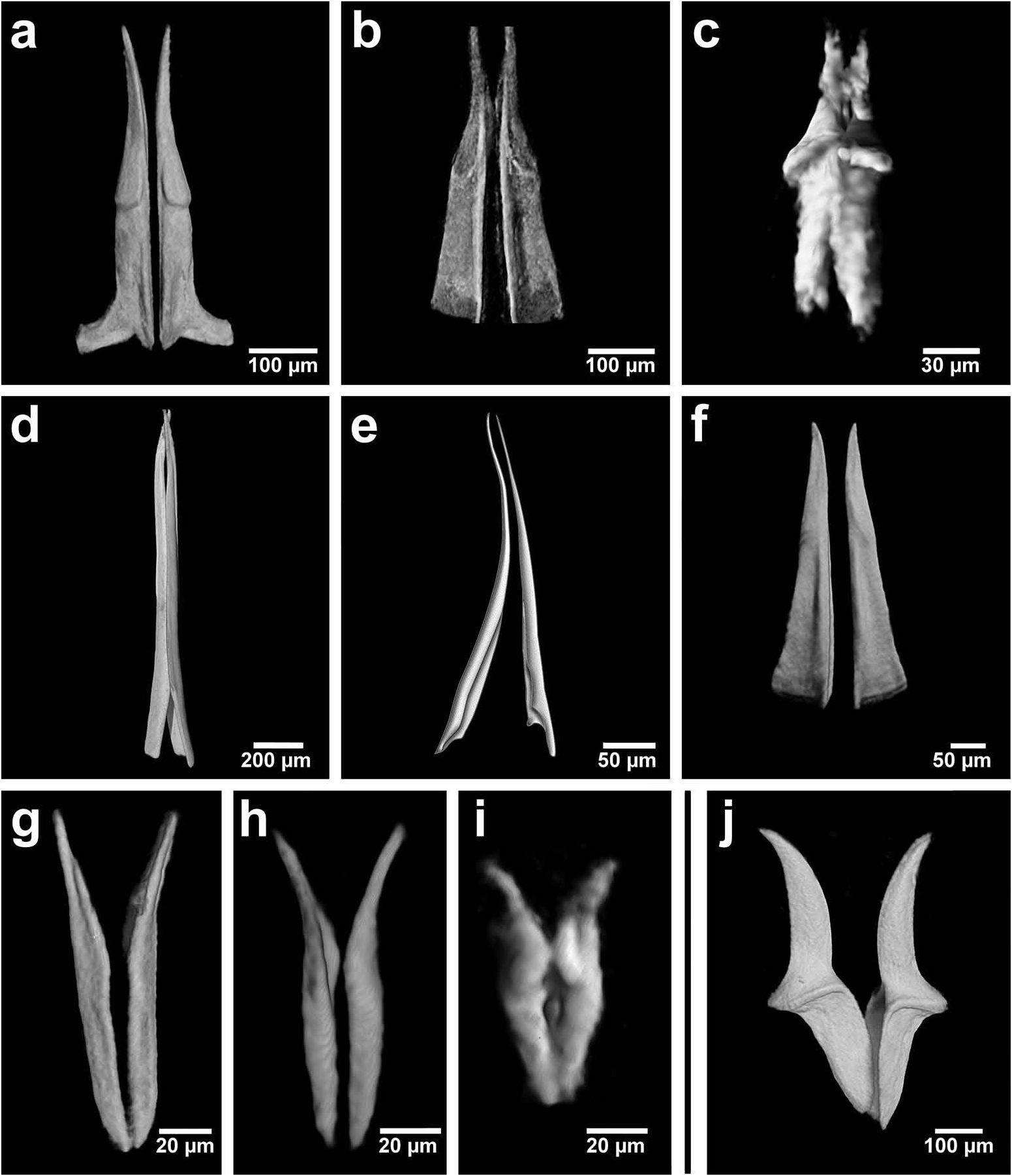
Based on the combination of morphological, sensory and buccal characters, nine out of 10 Chrysopetalinae genera fall into three distinct groups with each group sharing a similar jaw form; the exception being Strepternos ( Figure 23 View Figure 23 ).
Group 1. Chrysopetalum , Acanthopale and Thrausmatos ( Figure 23a–c View Figure 23 ) possess very pointed jaws diverging outwards at the distal third; a mid-way, diagonally sloping dorsal outer ridge and an inner longitudinal ridge. The very slender distal tips would allow penetration of prey tissue, serrations allowing even greater purchase, and jaw mid-way projections would hypothetically allow further anchoring of the embedded stylet tips. CT scans of Chrysopetalum and Acanthopale illustrate a flared basal jaw plate at the juncture of proboscis and pharynx, thereby offering greater stability (due to the enlarged surface connected to strong muscular tissue of the pharynx) to the entire stylet while everted for feeding.
The foot-like, basal jaw plate seen in Chrysopetalum stylets is more developed in comparison to the basal flared section seen in Acanthopale , although the shape of the proximal-most cross sections in both taxa are surprisingly similar. Chrysopetalum possesses diffuse muscle calcification in the anterior proboscidial area and a larger calcified section in the anterior pharynx, posterior to the jaws. There is a ‘granular’ band also seen in a similar position in the pharynx of Acanthopale which may be evidence of pharyngeal calcification. The proportions of the jaws relative to the size and form of the pharynx is most similar between Chrysopetalum and Acanthopale ; both are proportionally dissimilar to the relatively much shorter jaws and longer, partially differentiated pharynx of Thrausmatos . Strong internal calcification is seen in the jaws of Chrysopetalum , whereas Acanthopale and Thrausmatos jaws appear to share less dense internal calcification which is observed when comparing jaw cross sections between each taxon. A calcified pharyngeal muscle cannot be discerned in CT scans of Thrausmatos .
Group 1 taxa share the largest number of unique sensory characters in comparison with that of Groups 2 and 3, e.g. median antenna shape and position, number of tentacular cirri on segments 1 and 2 and a ‘free’ prostomium, i.e. not fused laterally to anterior segments. A discrete nuchal caruncle, ciliated and pigmented in live specimens, and retractile palp tips, also pigmented when live, are indicative that both structures possess a probable chemosensory function ( Lindsay 2009). Glandular pigmented ventral pads are present in taxa of Groups 1 and 2, but their structure and function need further clarification.
Larger species of Group 1 taxa possess a range of defensive dorsal cover types. Chrysopetalum and Acanthopale display a dorsal array of spiky, sabre-like and highly serrate, curved, slender notochaetal paleael types, respectively; Thrausmatos is covered by large fans of overlapping, symmetrical notochaetal paleae. Notochaetal paleae originating from multiple centres, as figured by Perkins (1985, figure 19A; this paper: Figure 8b View Figure 8 ) for Chrysopetalum species , is another unique character common to these three taxa. They also share flexible anterior segments that can leave the prostomium, complex eyes, sensitive long palps and a caruncular nuchal organ, open and free to engage in seeking out food, e.g. Chrysopetalum ( Figure 8b View Figure 8 ), Acanthopale ( Figure 2a View Figure 2 ).
Chrysopetalum , a speciose taxon occurring across a wide latitudinal and hard substrate range, contains small and larger species (5–20 mm L) which are morphologically different from each other, although the majority of these have also formed cryptic clades (Watson and Chatzigeorgiou, Forthcoming). Acanthopale (14 mm L) and Thrausmatos (30 mm L) are presently monotypic taxa; the former is found in shallow tropical reefs and the latter in the deep sea and associated with bivalve fauna at hydrothermal vents. Group 1 clades are epibenthic, relatively mobile compared to those of Groups 2 and 3, and can be found active among and feeding on invertebrate aggregations in shallower to deeper crevicular habitats.
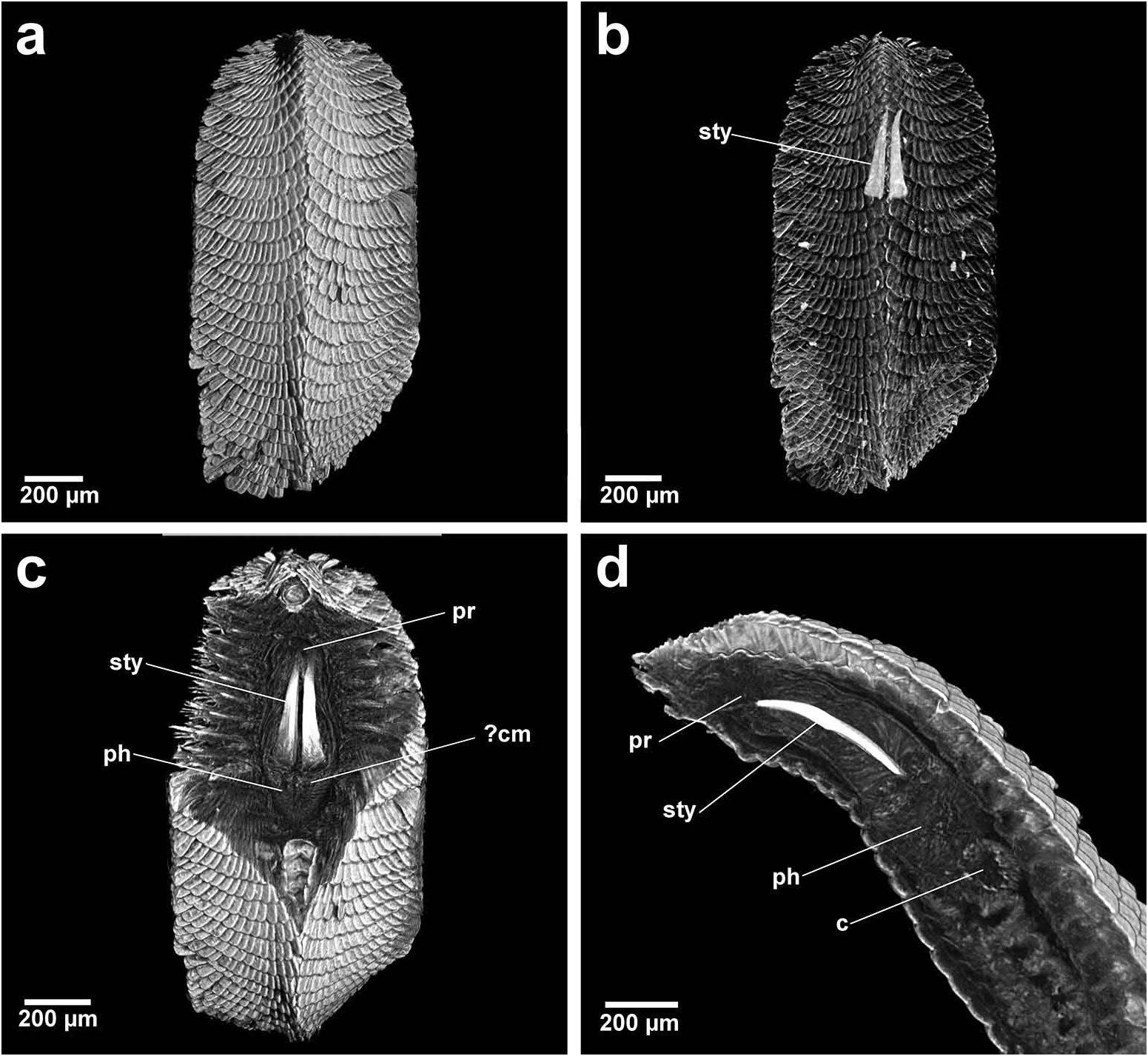
Group 2. Bhawania , Paleaequor and Arichlidon ( Figure 23d–f View Figure 23 ) possess a pair of pointed jaws that are positioned very close to each other along their entire length, evoking a powerful spear-like form that upon eversion would effectively pierce and find good traction in prey while feeding. Jaws have straight margins, do not diverge distally and possess a degree of basal flare; a large mid-way projection is absent.
Bhawania and Paleaequor possess a very similarly shaped, partially differentiated pharynx and the long, slender stylets of both taxa are most similar. Bhawania possesses broad, very robust, calcified jaws in comparison to the more slender, uncalcified jaws seen in Paleaequor . The strongly ‘kinked’ pharynx and associated complex glandular system observed in Paleaequor , however, are characters not seen in any other chrysopetalid taxa ( Figure 11b–d View Figure 11 ). Extensive pharyngeal glands, hypothetically possessing a secretive function, are considered structures intrinsic to feeding mode within Paleaequor species. Pharyngeal specializations may have evolved in response to preying on detritus feeders, i.e. ingesting the fluids of soft-skinned prey filled with mud that live in sedimentary habitats. Glands would clearly benefit from ultrastructural investigation and histological analysis to determine their exact structure and function.
Arichlidon is morphologically dissimilar when compared with the former taxa. It possesses a short, broad body and a short, broad, undifferentiated pharynx, closer to that seen in Chrysopetalum . The anterior pharynx also displays evidence of a ‘granular’ band, indicating possible calcification. Arichlidon jaws are comparatively shorter, broader and exhibit similar jaw cross sections to Chrysopetalum . Interestingly, Arichlidon also possesses a basally flared jaw and an inner longitudinal ridge ending in a spur: characters also seen in the jaws of Chrysopetalum species. Within the Chrysopetalinae these two taxa inhabit the broadest depth range and range of different crevicular habitats.
Bhawania and Paleaequor appear dependent on more specific crevicular habitats and possess protective sensory structures: very small prostomia fused with the anterior two segments in conjunction with a large retractile nuchal ridge and unique retractile dorsal styles within large cirrophores. Lateral organs and glandular ventral pads are also present. Arichlidon , the smallest taxon in Group 2 (2–5 mm L), possesses a more glandular nuchal fold and comparatively less prostomial retractability, and retractile dorsal cirri are lacking. However, all Group 2 taxa are distinguished by having the entire dorsum and prostomium covered by flattened notochaetal paleael types that originate in single fans forming an effective carapace, e.g. Arichlidon ( Figures 1a View Figure 1 and 4a View Figure 4 ); all taxa also share a similar formula of cirri of anterior segments.
Bhawania View in CoL , a longer bodied taxon (50 mm L), contains slow moving species that live at shallower depths in deeper crevicular spaces in hard and living substrates where they are able to flatten themselves to the shape of the crevice, tube, canal or polychaete host. Paleaequor species (18 mm L) are found in a very different habitat, among polychaete tube communities living in soft substrate muddy and sandy banks at shallow to moderate depths; Paleaequor View in CoL is the only Chrysopetalinae View in CoL taxon inhabiting soft substrates. Arichlidon species possess epitokous swimming neurochaetae and planktonic larvae which enables effective dispersal of species among the broadest range of biotopes across an extremely wide depth range (intertidal to abyssal) and in all habitat types, including inside the gills of molluscs and crustaceans. Group 2 taxa, especially Arichlidon View in CoL , are composed of cryptic species that are difficult to tell apart morphologically ( Watson Russell 1998, 2000a).
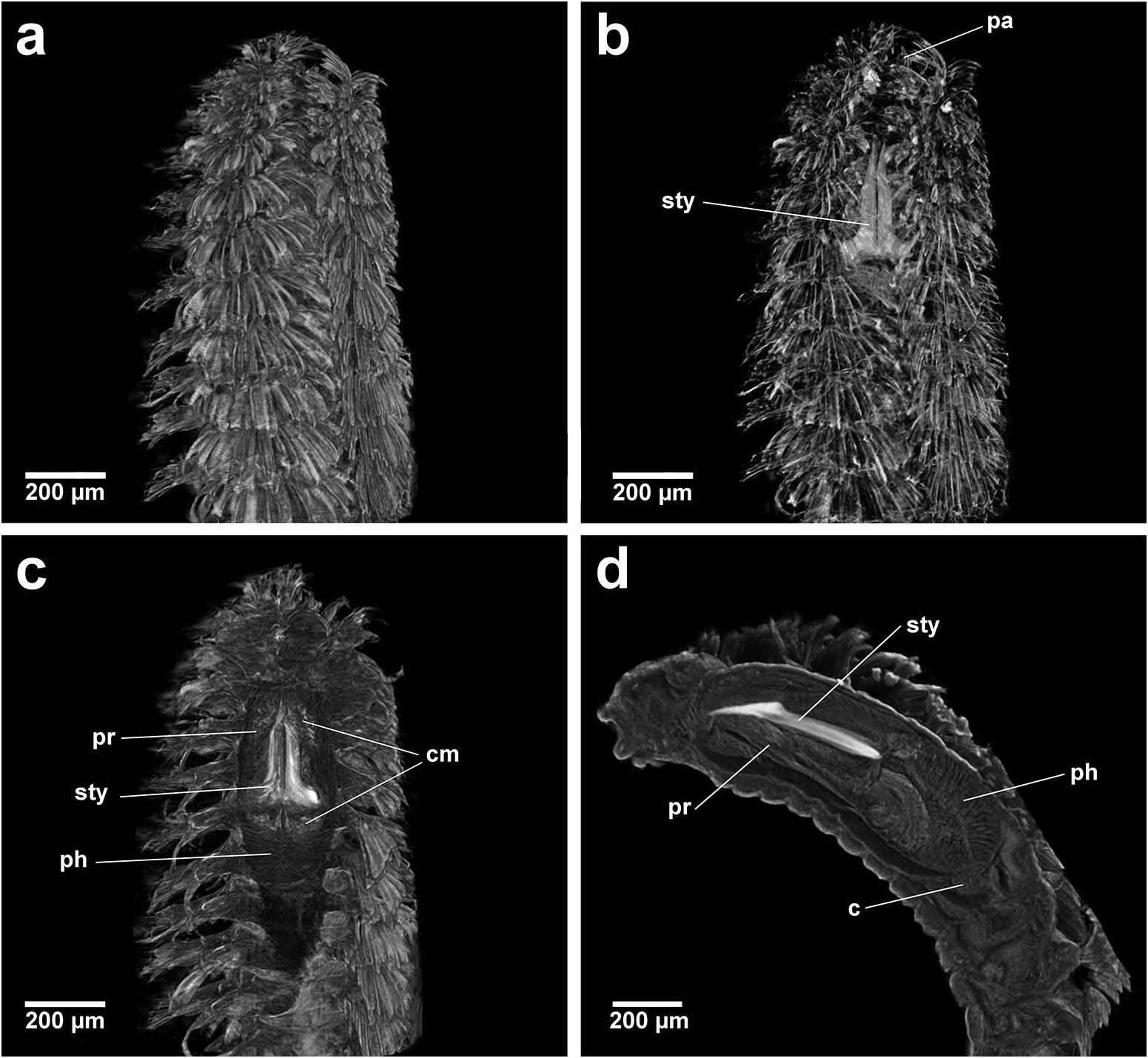
Group 3. Paleanotus View in CoL , Treptopale View in CoL and Hyalopale View in CoL ( Figure 23g –i View Figure 23 ) share a most homogeneously shaped jaw. In each taxon the two elongate stylets with slightly rounded tips diverge outwards at the distal half-way point; jaws display a slight inner mid-way projection and taper posteriorly with no basal flare or spur. The wide inner groove extends along the length of the jaw in Hyalopale View in CoL , whereas in Paleanotus View in CoL and Treptopale View in CoL the groove is present in the anterior 2/3 of the jaw. CT imaging does not indicate the proboscis as such a clearly defined structure in Group 3 taxa. Paleanotus View in CoL and Treptopale View in CoL stylets appear deeply embedded within the anterior pharynx; Hyalopale View in CoL jaws are a little more clearly evident at the proboscidial/pharynx juncture.
All Group 3 taxa possess a discrete pharyngeal calcified muscular ring that encircles the jaws at the mid-way point, being most developed in Paleanotus species. Among the Phyllodocida , only one reference is found describing a comparable specialized pharynx: that of the ectoparasitic syllid, Parasitosyllis Potts, 1912 . Its ‘chitinized’ vase-shaped pharynx, composed of four layers, is described as extending into and becoming increasingly and firmly embedded in prey with a thin proximal channel enabling passage of fluids from the host ( Potts 1912; Martin and Britayev 1998).
The mechanics of the Paleanotus calcified pharyngeal ring in feeding is uncertain but it is hypothesized it would function in similar ways to that seen in Parasitosyllis . The Paleanotus everted proboscis with terminal papillae makes contact with prey and the muscular force of the pharynx retracts the proboscis in part, driving the calcified ring forward to embed in tissue, thereby allowing greater traction for the stylets to move forward and enabling more stable penetration. The presence of tanned, roughened, distal stylet tips would also effect a rasping action and initiate better penetration into tissue. The muscular pharynx would enable pulses of sucking motion allowing fluids to be extracted from prey and subsequently passing down the internal groove of the stylets.
Treptopale View in CoL (10 mm length) and the very small taxon Hyalopale View in CoL (<3 mm L) share a similar shaped undifferentiated pharynx and a less-developed calcified pharyngeal ring. Both taxa are often found associated with dead coral and calcareous algae with dark green material frequently observed in the gut. Herbivory was considered the probable feeding mode ( Watson 2010). In light of re-evaluation of buccal structure in this study (muscular pharynx and calcified muscle, stylet jaw structure including tanned jaw tips); the integrated feeding function (piercing, traction and sucking); and the need for ‘quick, rich protein hits’, it is hypothesized that these particularly small taxa feed opportunistically on micro-invertebrates that live and feed amongst algae. Interestingly, small species of Paleanotus View in CoL (<5 mm) also frequently exhibit dark green guts ( Watson 2015).
Sensory characters in Paleanotus View in CoL and the related taxon Treptopale View in CoL include a small nuchal fold in conjunction with a moderately retractile prostomium fused with the anterior two segments, two pairs of large eyes, pair of ventral oval palps and particularly well-developed lateral organs. Hyalopale View in CoL possesses similar characters but the nuchal fold is very diminished to absent. The dorsum and prostomium in all Group 3 taxa are completely covered by flattened paleael notochaetal types, e.g. Treptopale View in CoL ( Figure 17a View Figure 17 ). Specimens of Group 3 taxa are much more likely to automatically fragment when handled in comparison to those of in Groups 1 and 2.
Paleanotus species are present across a widespread range of latitudes and different habitats and frequently found among fouling faunas, including inside mollusc and crustacean gills. Paleanotus View in CoL comprises slender, very small and moderate-sized, slow moving species (3–15 mm L) which are morphologically different from each other, as well as some species forming cryptic complexes ( Watson, 2015). All species within the genus Treptopale View in CoL exhibit cryptic morphology, e.g. a recent molecular study of two former nominal species from northern Australia revealed nine clades ( Wei et al. 2013). Morpho-species of Hyalopale View in CoL exhibit a similar crypticism. The very small size at maturity of Hyalopale View in CoL , comparative lack of body cover, and its endo-/epibenthic habitats indicates a meiofaunal lifestyle (Watson and Rouse, in prep.).
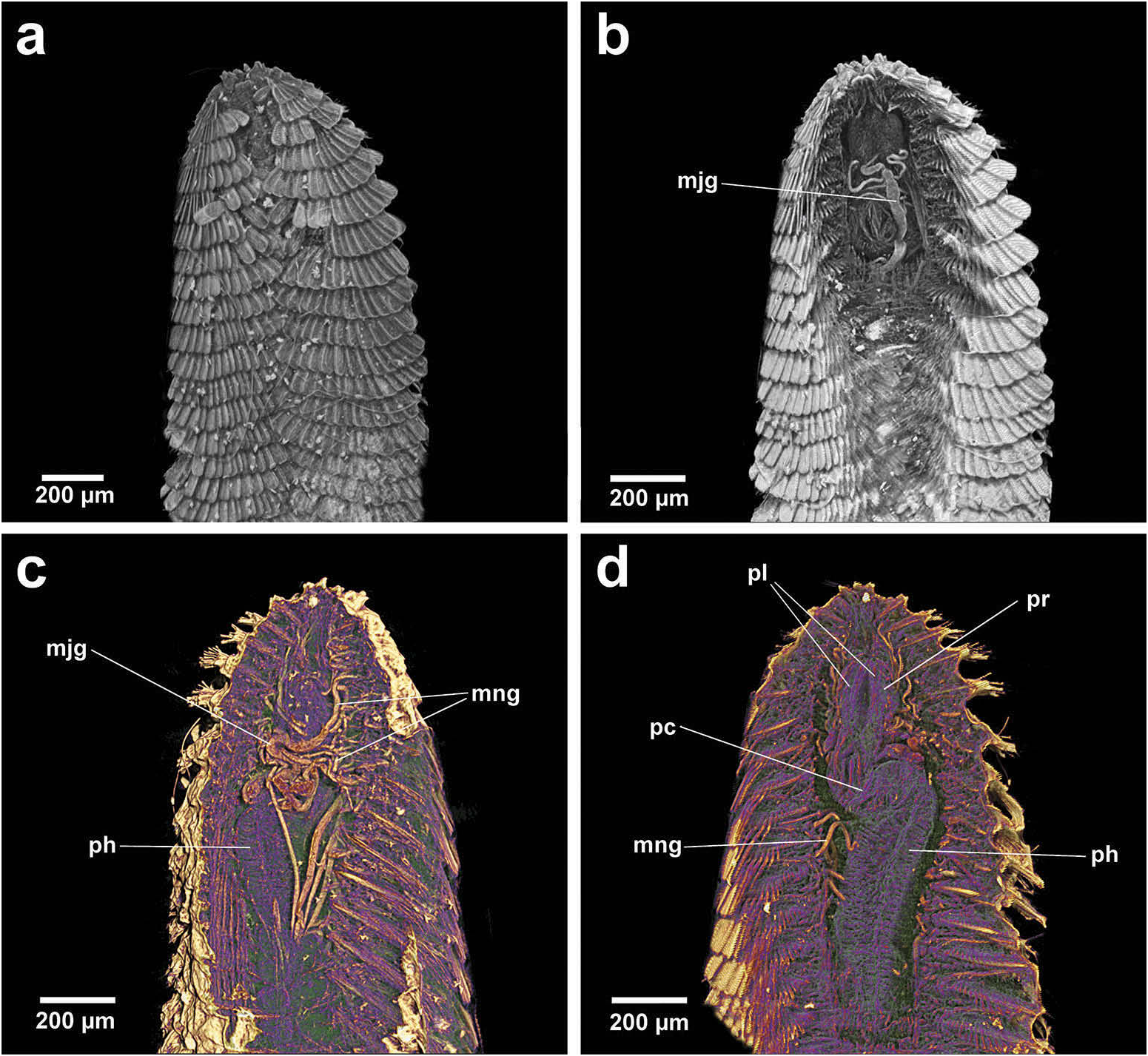
Position of Strepternos View in CoL . On the basis of morphology, the jaws of Strepternos didymopyton View in CoL appear at first most similar to those of the deep-sea taxon Thrausmatos View in CoL of Group 1 (compare Figure 23c, j View Figure 23 ). Stylet jaws of both taxa exhibit a large mid-way projection and the posterior jaw halves partially interlink. However, the comparative body morphology of both taxa is quite different, including the nuchal organ shape, number of cirri of the anterior segments and chaetal insertion patterns ( Table 2); details of the mid-way ridge, anterior angle of the jaws and particularly body morphology align Thrausmatos View in CoL more closely with Group 1 taxa.
Strepternos jaws appear the most robust, heavily calcified and broadest compared with all other Chrysopetalinae taxa. The anterior jaw halves diverge in a manner similar to those taxa of Group 3, but there is a large difference in the development of the midway ridge. The posterior jaw halves that interlink in Strepternos is observed, as mentioned in Thrausmatos , and also in Bhawania , Group 2. Strepternos shares with Bhawania a more similar body morphology: a particularly broad anterior end with a very small, retractile prostomium in concert with a nuchal ridge, similar shaped head appendages and partly retractile styles within large dorsal cirrophores ( Watson Russell 1991).
The projecting shelf on the outer jaw margin of Strepternos occurs at the proboscidial/pharynx juncture, enabling firm anchoring of the jaws while the posterior halves of the stylets extend down into the anterior pharynx, ensuring greater stability and ‘sticking power’ when everted and feeding. The particular anterior angle of the two stylet jaws, with thickened and serrated tips, looks ideal for splitting open xylophagid valves. Turner (1978, p. 16) observed chrysopetalid (= Strepternos ) polychaetes between xylophagid valves ‘obviously feeding on Xylophaga ’.
Large numbers of Strepternos didymopyton planktonic larvae have been described settling on experimental wood islands, and adults have only ever been recovered from deep-sea wood substrates, with male and female individuals often found dorsum to dorsum inside empty xylophagid tubes ( Watson Russell 1991, 1997). Strepternos appears to be an active carnivore occupying a specialized niche.
Based primarily on body morphology analysis, Strepternos is provisionally considered part of Group 2. Conversely, Strepternos and Thrausmatos may be related deep-sea clades but their similar jaw morphology may be a convergence reflective of adaption to a bivalve food source.
Summary of subfamily Chrysopetalinae . The subfamily Chrysopetalinae exhibits the greatest number and diversity of clades and range of sensory characters allied with diversity of pharyngeal and jaw structures. The differentiated pharynx is only found in larger taxa of the Chrysopetalinae and, interestingly, is represented in each of the three designated groups, reflective of the fact that all contain lineages of larger individuals. Morphological differences are observed in jaws between groups of taxa and not at the species level. This generality of jaw morphology points to a shared specific feeding function. All 10 Chrysopetalinae taxa display the functionality of a piercing jaw morphology in concert with sucking of fluids from prey using a highly muscular pharynx. Jaw form, sensory characters and feeding mode coupled with habitat suggests free-living, opportunistic carnivory, extending to semi-parasiticism.
Jumars et al. (2015) consider the single pair of chrysopetalid stylets as laterally opposed and are therefore described as holding prey. The present study, using micro- CT imaging, has revealed that taxa of Groups 1–3 and Strepternos possess stylets that are individually grooved: either deeply along the entire length, e.g. Bhawania , or more shallowly with solid distal tips, e.g. Chrysopetalum . The pair of grooved stylets is situated very close up against each other and the space formed in-between would in effect constitute a tube. Pointed stylet jaws interlock, either along the entire or most of their length, and, rather than holding, appear capable of acting as a single co-ordinated unit with a primary action of piercing and gaining traction within prey tissues. Mid-jaw or basal enlargements would provide additional stability for jaw injection or serve as an anchoring device to attach to a host after piercing.
It is hypothesized that upon partial eversion of the proboscis and ‘feeling out’ of potential prey tissue by terminal papillae, the muscular pharynx initiates swift action with powerful contractions propelling the pointed jaws forward and puncturing prey tissue; further muscular movements enables a sucking motion to effect transport of prey fluids down the stylet groove. It is very possible that membrane disruptive enzymes are also transported along this hollow groove (Watson et al., in prep.).
Specialized pharyngeal modifications are represented in varying degrees within taxa of Groups 1–3; similarities may be indicative of homoplasy, or as we posit, reflect deeper phylogenetic relationships. Calcified pharyngeal muscle, for example, is most developed in Group 3, and also present in some taxa within Groups 1 and 2 – a first observation within the Chrysopetalidae . Its presence throughout the Chrysopetalinae suggests taxa may have had periods of a more specialized ectoparasitic mode of life in the past where causal events supported phenotypic modifications of the pharynx. Chrysopetalinae taxa are essentially considered free-living and, in the present, appear to have the flexibility to adopt a successful facultative symbiont mode of life where opportunity and need be.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Chrysopetalinae
| Watson, Charlotte & Faulwetter, Sarah 2017 |
Thrausmatos
| Watson 2001 |
Thrausmatos
| Watson 2001 |
Arichlidon
| Watson Russell 1998 |
Strepternos
| Watson Russell 1991 |
Paleaequor
| Watson Russell 1986 |
Treptopale
| Perkins 1985 |
Hyalopale
| Perkins 1985 |
Hyalopale
| Perkins 1985 |
Treptopale
| Perkins 1985 |
Treptopale
| Perkins 1985 |
Hyalopale
| Perkins 1985 |
Treptopale
| Perkins 1985 |
Hyalopale
| Perkins 1985 |
Treptopale
| Perkins 1985 |
Hyalopale
| Perkins 1985 |
Treptopale
| Perkins 1985 |
Treptopale
| Perkins 1985 |
Hyalopale
| Perkins 1985 |
Hyalopale
| Perkins 1985 |
Chrysopetalinae
| Ehlers 1864 |
Bhawania
| Schmarda 1861 |
Paleanotus
| Schmarda 1861 |
Paleanotus
| Schmarda 1861 |
Paleanotus
| Schmarda 1861 |
Paleanotus
| Schmarda 1861 |
Paleanotus
| Schmarda 1861 |
Paleanotus
| Schmarda 1861 |