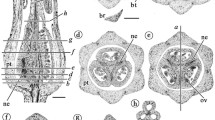
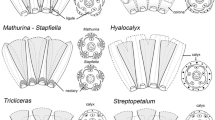
Abstract
Floral features contribute with remarkable additions to morphological studies and are widely used to address questions about the evolution and diversification of several groups of plants. Even though Simaroubaceae are a small monophyletic family, the few detailed structural analyses of reproductive organs and the floral diversity and variations already described in their members stimulate novel structural studies. In this study, we investigate the evolution of reproductive features of Simaroubaceae by means of a combination of original data and a review of the literature, aiming to elucidate which floral characters are most informative for a better understanding of the evolutionary history of the group. We analyzed 21 out of the 23 genera of Simaroubaceae, plus six from Rutaceae and seven from Meliaceae as outgroups. We used a Bayesian method and the Parsimony optimality criterion to reconstruct ancestral reproductive character states using a re-analyzed phylogenetic tree of Sapindales. Here, we combined available molecular sequences to have the largest sample of Simaroubaceae genera. We found that the ancestral flowers of Simaroubaceae were probably polygamous or dioecious plants, with free carpels united only distally, with divergent, elongated stigmas, and with drupaceous, laterally flattened to lenticular fruits. The latter feature plus apocarpous carpels are putative synapomorphies of the family retrieved in this study. Imbricate petals and a diplostemonous androecium were recovered as conditions found in the ancestor of Simaroubaceae but also shared with the ancestors of Meliaceae and Rutaceae. Our findings were mostly in accordance with previous evolutionary studies on genera of Simaroubaceae and with other families of Sapindales.











Similar content being viewed by others
References
Abbe EC, Earle TT (1940) Floral anatomy and morphology of Leitneria floridana. J Torrey Bot Soc 67:173–193
Alves GGN, El Ottra JHL, Devecchi MF, Demarco D, Pirani JR (2017) Structure of the flower of Simaba (Simaroubaceae) and its anatomical novelties. Bot J Linn Soc 183:162–176
Armbruster WS, Debevec EM, Willson MF (2002) Evolution of syncarpy in angiosperms: theoretical and phylogenetic analyses of the effects of carpel fusion on offspring quantity and quality. Int J Evol Biol 15:657–672
Aubréville A (1962) Flora du Gabon Irvingiacées, Simaroubacées, Burséracées. Mus Natl Hist Nat 3:3–99
Avalos A, Zini LM, Ferruci MS, Lattar EC (2019) Anther and gynoecium structure and development of male and female gametophytes of Koelreuteria elegans subsp. formosana (Sapindaceae): phylogenetic implications. Flora 255:98–109
Bachelier JB, Endress PK (2008) Floral structure of Kirkia (Kirkiaceae) and its position in Sapindales. Ann Bot 102:539–550
Bachelier JB, Endress PK (2009) Comparative floral morphology of Anacardiaceae and Burseraceae, with a special emphasis on the gynoecium. Bot J Linn Soc 159:499–571
Bachelier JB, Endress PK, De Craene LPR (2011) Comparative floral structure and development of Nitrariaceae (Sapindales) and systematic implications. In: Wanntorp L, De Craene LPR (eds) Flowers on the tree of life. Cambridge University Press, Cambridge, pp 181–217
Baratake RC, Patil CG (2010) Cytological investigations in poly–gamo–dioecious tree Simarouba glauca DC. Nucleus 53:33–36
Barrett SCH (2002) The evolution of plant sexual diversity. Nat Rev 3:274–284
Barrett SCH, Hough J (2013) Sexual dimorphism in flowering plants. J Exp Bot 64:67–82
Bawa KS, Beach JH (1981) Evolution of sexual systems in flowering plants. Ann Mo Bot Gard 68:254–274
Beurton CH (1994) Gynoecium and perianth in Zanthoxylum s.l. (Rutaceae). Plant Syst Evol 189:165–191
Blume CL (1825) Picrasma. In: Bijdragen tot de flora van Nederlandsch Indië. Ter Lands Drukkerij, Batavia, pp 247–248
Bolmgren K, Eriksson O (2010) Seed mass and the evolution of fleshy fruits in angiosperms. Oikos 119:707–718
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A (2019) BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol 15:e1006650
Bremer B, Eriksson O (1992) Evolution of fruit characters and dispersal modes in the tropical family Rubiaceae. Biol J Linn Soc 47:79–95
Capuron R (1961) Cotribution à l’étude de la flore florestière de Madagascar. Adansonia 1:65–92
Caris P, Smets E, De Coster K, Ronse De Craene LP (2006) Floral ontogeny of Cneorum tricoccon L. (Rutaceae). Plant Syst Evol 257:223–232
Cavalcante PB (1983) Revisão taxonômica do gênero Simaba Aubl. (Simaroubaceae) na América do Sul. Bol Mus Para Emilio Goeldi 37:1–85
Channell RB, Wood CE (1962) The Leitneriaceae in the southeastern United States. J Arnold Arbor 18:435–438
Charlesworth B, Guttman DS (1999) The evolution of dioecy and plant sex chromosome systems. In: Ainsworth CC (ed) Sex determination in plants. Bios Scientific, Oxford, pp 25–49
Clayton JW, Fernando ES, Soltis PS, Soltis DE (2007) Molecular phylogeny of the Tree–of–Heaven family (Simaroubaceae) based on chloroplast and nuclear markers. Int J Plant Sci 168:1325–1339
Clayton JW (2011) Simaroubaceae. In: Kubitzki K (ed) The families and genera of vascular plants. Vol X. Flowering plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae. Springer, Berlin, pp 408–423
Clifford HT, Dettmann ME (2001) Drupe – a term in search of a definition. Austrobaileya 6:127–131
Cronquist A (1944a) Studies in the Simaroubaceae III. The Genus Simaba. Lloydia 7:81–92
Cronquist A (1944b) Studies in the Simaroubaceae. IV. Resume of the American Genera. Brittonia 5:128–147
Cronquist A (1981) An integrated system of classification of flowering plants. Columbia University Press, New York
Darwin C (1877) The different forms of flower plants on plants of the same species. John Murray, London
Devecchi MF, Pirani JR (2020) Flora do Espírito Santo: Simaroubaceae. Rodriguésia 71:e02942018
Devecchi MF, Thomas WW, Pluckett GM, Pirani JR (2018a) Testing the monophyly of Simaba (Simaroubaceae): Evidence from five molecular regions and morphology. Mol Phylogenetics Evol 120:63–82
Devecchi MF, Thomas WW, Pirani JR (2018b) Taxonomic revision of the neotropical genus Homalolepis Turcz. (Simaroubaceae). Phytotaxa 366:1–108
El Ottra JHL, Pirani JR, Endress PK (2013) Fusion within and between whorls of floral organs in Galipeinae (Rutaceae): structural features and evolutionary implications. Ann Bot 111:821–837
El Ottra JHL, Pirani JR, Pansarin ER (2016) Floral biology and pollination of two sympatric species of Galipeinae (Galipeeae, Rutaceae) endemic to the Brazilian Atlantic Forest. Flora 221:107–116
El Ottra JHL, Demarco D, Pirani JR (2019) Comparative floral structure and evolution in Galipeinae (Galipeeae: Rutaceae) and its implications at different systematic levels. Bot J Linn Soc 191:30–101
Endress PK (1994) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, Cambridge
Endress PK (2010a) Synorganization without organ fusion in the flowers of Geranium robertianum (Geraniaceae) and its not so trivial obdiplostemony. Ann Bot 106:678–695
Endress PK (2010b) Flower structure and trends of evolution in eudicots and their major clades. Ann Mo Bot Gard 97:541–583
Endress PK (2016) Development and evolution of extreme synorganization in angiosperm flowers and diversity: a comparison of Apocynaceae and Orchidaceae. Ann Bot 117:749–767
Endress PK, Doyle JA (2015) Ancestral traits and specializations in the flowers of the basal grade of living angiosperms. Taxon 64:1093–1116
Endress PK, Matthews ML (2006) First steps towards a floral structural characterization of the major rosid subclades. Plant Syst Evol 260:223–251
Endress PK, Jenny M, Fallen ME (1983) Convergent elaboration of apocarpous gynoecia in higher advanced dicotyledons (Sapindales, Malvales, Gentianales). Nord J Bot 3:293–300
Engler A (1931a) Simaroubaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol 19a, 2nd edn. Engelmann, Leipzig, pp 359–405
Engler A (1931b) Rutaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol 19a, 2nd edn. Engelmann, Leipzig, pp 187–359
Engler A (1931c) Burseraceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol 19a, 2nd edn. Engelmann, Leipzig, pp 405–456
Faegri L, van der Pijl L (1979) The principles of pollination ecology, 3rd edn. Pergamon Press, New York
Fernando ES, Gadek PA, Quinn CJ (1995) Simaroubaceae, an artificial construct: evidence from rbcL sequence variation. Am J Bot 82:92–103
Forman L, Bridson D (1992) The Herbarium Handbook. Royal Botanic Gardens, Kew, London
Franceschinelli EV, Thomas WW (2000) Simaba guianensis subsp. huberi, a new Venezuelan taxon of Simaroubaceae. Brittonia 52:311–314
Gadek PA, Fernando ES, Quinn CJ, Hoot SB, Terrazas T, Sheahan MC, Chase MW (1996) Sapindales: molecular delimitation and infraordinal groups. Am J Bot 83:802–811
Gama RL, Muellner-Riehl AN, Demarco D, Pirani JR (2021a) Evolution and reproductive traits in the mahagony family (Meliaceae). J Syst Evol 59:21–43
Gama RL, El Ottra JHL, Pirani JR, Demarco D (2021b) Gynodioecy in Trichilia (Meliaceae) and a peculiar case of male sterility due to tapetum secretion. Braz J Bot. https://doi.org/10.1007/s40415-021-00746-4
Goldberg EE, Otto SP, Vamosi JC, Mayrose I, Sabath N, Ming R, Ashman TL (2017) Macroevolutionary synthesis of flowering plant sexual systems. Evolution 71:898–912
Gottsberger G (1978) Seed dispersal by fish in the inundated regions of Humaita, Amazonia. Biotropica 10:170–183
Gouvêa CF, Dornelas MC, Rodriguez APM (2008) Floral development in the tribe Cedreleae (Meliaceae sub–family Swietenioideae): Cedrela and Toona. Ann Bot 101:39–48
Groppo M, Kallunki JA, Pirani JR, Antonelli A (2012) Chilean Pitavia more closely related to Oceania and Old World Rutaceae than Neotropical groups: evidence from two cpDNA non–coding regions, with a new subfamilial classification of the family. PhytoKeys 19:9219
Gut BJ (1966) Beiträge zur Morphologie des Gynoeciums und der Blütenachse einiger Rutaceen. Bot Jahrb Syst 85:151–247
Hardesty BD, Dick CW, Kremer A, Hubbell S, Bermingham E (2005) Spatial genetic structure of Simarouba amara Aubl. (Simaroubaceae), a dioecious, animal-dispersed Neotropical tree, on Barro Colorado Island. Panama J Hered 95:290–297
Harms H (1896) Meliaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol 19a, 2nd edn. Engelmann, Leipzig, pp 258–308
Heilbuth J (2000) Lower species richness in dioecious clades. Am Nat 156:221–241
Honda EMS (1974) Contribuição ao conhecimento da biologia de peixes do Amazonas. II – Alimentação de tambaqui, Colossoma bidens (Spix). Acta Amaz 4:47–53
Hu SY (1979) Ailanthus. Arnoldia 39:29–50
Janzen DH (1979) New horizons in the biology of plant defenses. In: Rosenthal GA, Janzen DH (eds) Herbivores their interaction with secondary plant metabolites. Academic Press, Orlando, pp 331–350
Judd WS, Campbell CS, Kellog EA, Stevens PF, Donoghue MJ (2008) Plant systematics. A phylogenetic approach, 3rd edn. Sinauer Associates, Sunderland
Kubitzki K (2011) Introduction to Sapindales. In: Kubitzki K (ed) The families and genera of vascular plants, vol X. Springer, Heildelberg, pp 1–3
Kubitzki K, Gottlieb O (1984) Micromolecular patterns and the evolution and major classification of angiosperms. Taxon 33:375–391
Kubitzki K, Kallunki JA, Duretto M, Wilson P (2011) Rutaceae. In: Kubitzki K (ed) The families and genera of vascular plants, vol X. Springer, Heildelberg, pp 276–356
Leins P, Erbar C (2010) Flower and fruit. Morphology ontogeny phylogeny function and ecology. Schweizerbart Science Publisher, Stuttgart
Lin N, Moore MJ, Deng T, Sun H, Yang L, Yan S, Wang H (2018) Complete plastome sequencing from Toona (Meliaceae) and phylogenomic analyses within Sapindales. Appl Plant Sci 6:1–11
Lloyd DG, Bawa KS (1984) Modification of the gender of seed plants in varying conditions. Evol Biol 17:255–338
Lorts CM, Briggeman T, Sang T (2008) Evolution of fruit types and seed dispersal: a phylogenetic and ecological snapshot. J Sys Evol 46:396–404
Mabberley DJ (2011) Meliaceae. In: Kubitzki K (ed) The families and genera of vascular plants, vol X. Springer, Berlin, pp 185–211
Maddison WP, Maddison DR (2019) Mesquite: a modular system for evolutionary analysis. Version 3.61. Available from http://mesquiteproject.org. Accessed in 2019 and 2020
Majure LC, Clase T, Blankenship A, Noa-Monzón A (2021a) A new species of Picrasma, P. nanophylla (Simaroubaceae), from the Dominican Republic. Brittonia. https://doi.org/10.1007/s12228-021-09656-x
Majure LC, Pham K, Clase T (2021b) Castela senticosa (Simaroubaceae: Sapindales), a new species of the Greater Antillean clade endemic to Hispaniola. Syst Bot (in press)
Matthews ML, Amaral MCE, Endress PK (2012) Comparative floral structure and systematics in Ochnaceae s.l. (Ochnaceae, Quiinaceae, Medusagynaceae; Malpighiales). Bot J Linn Soc 170:299–392
Minelli A (2018) Plant evolutionary developmental biology. The evolvability of the phenotype. Cambridge University Press, Cambridge
Moran R, Felger R (1968) Castela polyandra, a new species in a new section; union of Holacantha with Castela (Simaroubaceae). Trans San Diego Soc Nat Hist 15:31–40
Muellner-Riehl NA, Clayton JW, Buerki S, Nauheimer L, Chiang YC, Cody S, Pell SK (2016) Molecular phylogenetics and molecular clock dating of Sapindales based on plastid rbcL, atpB and trnL–trnF DNA sequences. Taxon 65:1019–1036
Naghiloo S, Classen-Bockhoff R (2016) Developmental analysis of merosity and sexual morphs in Rubiaceae: a case study in Rubia and Cruciata. Flora 222:52–59
Nair NC, Joshi RK (1958) Floral morphology of some members of the Simaroubaceae. Bot Gaz 120:88–99
Noa-Monzón A, Gutiérrez PAG (2019) Picrasma pauciflora (Simaroubaceae), a new species from the NE coast of Cuba. Willdenowia 49:187–191
Noteboom HP (1962a) Simaroubaceae. Flora Malesiana (ser I) 6:193–226
Noteboom HP (1962b) Generic delimitation in Simaroubaceae tribe Simaroubae and a conspectus of the genus Quassia L. Blumea 9:509–528
Noteboom HP (1987) Laumoniera, a new genus of Simaroubaceae from Sumatra. Blumea 32:383–384
Pagel M, Meade A (2006) Bayesian analysis of correlated evolution of discrete characters by reversible–jump Markov chain Monte Carlo. Am Nat 167:808–825
Pannell JR (2017) Plant sex determination. Curr Biol 27:191–197
Paulino JV, Mansano VF, Prenner G, Teixeira SP (2017) High developmental lability in the perianth of Inga (Fabales, Fabaceae): a Neotropical woody rosid with gamopetalous corolla. Bot J Linn Soc 183:146–161
Pennington TD, Styles BT (1975) A generic monograph of the Meliaceae. Blumea 22:419–540
Pirani JR (1987b) Flora da Serra do Cipó, Minas Gerais: Simaroubaceae. Bol Bot USP 9:219–226
Pirani JR (1998) A revision of Helietta and Balfourodendron (Rutaceae, Pteleinae). Brittonia 50:348–380
Pirani JR, El Ottra JHL, Menezes NL (2010) Morfoanatomia da flor de cinco espécies de Galipea Aubl. e seu significado na evolução das flores tubulosas entre as Rutaceae neotropicais. Rev Bras Bot 32:301–318
Pirani JR, Majure LC, Thomas WW, Devecchi MF (2021) An updated account of Simaroubaceae with emphasis on American taxa. Braz J Bot. https://doi.org/10.1007/s40415-021-00731-x
Pirani JR (1987a) Simaroubaceae. In: Spichiger R (ed) Flora del Paraguay. Conservatoire et Jardin botaniques de la Ville Genève. Missouri Botanical Garden, Saint Louis
Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E (2007) Evolution and development of inflorescence architectures. Science 316:1452–1456
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904
Ramp E (1988) Struktur, Funktion und systematische Bedeutung des Gynoeciums bei den Rutaceae und Simaroubaceae. PhD dissertation, University of Zurich, Zurich
Rendle AB (1952) The classification of flowering plants. II. Dicotyledons. Cambridge University Press, Cambridge
Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am J Bot 101:1588–1596
Romero-da-Cruz MV, Guimarães R, Devecchi MF, Pirani JR, Forni-Martins ER (2021) Chromosome numbers in Homalolepis Turcz and their significance in Simaroubaceae evolution. Braz J Bot. https://doi.org/10.1007/s40415-021-00729-5
Ronse De Craene LP (2010) Floral diagrams. Cambridge University Press, Cambridge
Ronse De Craene LP, Bull-Hereñu K (2016) Obdiplostemony: the occurrence of a transitional stage linking robust flower configurations. Ann Bot 117:709–724
Ronse De Craene LP, Smets E (1995) The distribution and systematic relevance of the androecial character oligomery. Bot J Linn Soc 118:193–247
Ronse De Craene LP, Smets E (2001) Staminodes: their morphological and evolutionary significance. Bot Rev 67:351–390
Ronse De Craene LP, Smets E (2016) Meristic changes in flowering plants: how flowers play with numbers. Flora 221:22–37
Roubik DW, Holbrook NM, Parra GV (1985) Roles of nectar robbers in reproduction of the tropical treelet Quassia amara (Simaroubaceae). Oecologia 66:161–167
Sakai AK, Weller SG (1999) Gender and sexual dimorphism in flowering plants: A review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 1–31
Schilep KP (2011) Phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593
Schrader JA, Graves WR (2011) Taxonomy of Leitneria (Simaroubaceae) resolved by ISSR, ITS, and morphometric characterization. Castanea 76:313–338
Shu KM (2008) Picrasma. In: Flora of China. eFloras. Available from http://www.efloras.org. Accessed on 20 August 2019
Simpson MG (2010) Plant systematics, 2nd edn. Elsevier, Amsterdam
Sokoloff DD, Nuraliev MS, Oskolski AA, Remizowa MV (2018) Gynoecium evolution in angiosperms: monomery, pseudomonomery and mixomery. Moscow Univ Biol Sci Bull 72:97–108
Soltis PS, Brockington SF, Yoo MJ, Piedrahita A, Latvis M, Moore MJ, Chanderbali AS, Soltis DE (2009) Floral variation and floral genetics in basal angiosperms. Am J Bot 96:110–128
Stevens PF (2001) onwards. Angiosperm Phylogeny Website. Version 14, July 2017 [and more or less continuously updated since]. Available from http://www.mobot.org/MOBOT/research/APweb/
Stuessy TF (2009) Plant taxonomy: the systematic evaluation of comparative data. Columbia University Press, Ney York
Styles BT (1972) The flower biology of the Meliaceae and its bearing on tree breeding. Silvae Genet 21:175–182
Takhtajan A (1997) Diversity and classification of flowering plants. Columbia University Press, New York
Thomas WW (1990) The American genera of Simaroubaceae and their distribution. Acta Bot Bras 4:11–18
Tobe H (2011) Embryological evidence supports the transfer of Leitneria floridana to the family Simaroubaceae. Ann Mo Bot Gard 98:277–293
Tobe H (2013) Morphology and structure of staminate inflorescences and flowers of Leitneria floridana (Simaroubaceae): revisited. Acta Phytotaxon Geobot 63:57–62
Tölke ED, Demarco D (2020) The development of pseudomonomerous gynoecia: Anacardiaceae (subfamily Anacardioideae) as a case study. In: Demarco D (ed) Plant ontogeny. Nova Science, New York, pp 232–262
Walker-Larsen J, Harder LD (2000) The evolution of staminodes in angiosperms: patterns of stamen reduction, loss, and functional re–invention. Am J Bot 87:1367–1384
Weberling F (1989) Morphology of flowers and inflorescences. Cambridge University Press, Cambridge
Wei L, Xiang XG, Wang YZ, Li ZY (2015) Phylogenetic relationship and evolution of the androecia in Ruteae (Rutaceae). PLoS ONE 10:1–14
Willis JC (1951) A dictionary of the flowering plants and ferns. Cambridge University Press, Cambridge
Yadav N, Pandey A, Bhatnagar A (2016) Cryptic monoecy and floral morph types in Acer oblongum (Sapindaceae): An endangered taxon. Flora 224:183–190
Acknowledgements
This study is part of the PhD dissertation of the first author. We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; proc. #2014/18002‐2), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; proc. #307655/2015‐6) and International Association for Plant Taxonomy (IAPT) for assistance in funding research. We are most grateful to Dr. Alexandra N. Muellner-Riehl for providing the calibrated tree of Sapindales, to Klei Rodrigo Sousa for flower illustrations, Allan Bruno Sendas for enlightenments in figure editing, Dr. J. Richard Abbott, Dr. W. Wayt Thomas and Dr. Pedro Acevedo-Rodriguez for sharing images of Simaroubaceae representatives.
Author information
Authors and Affiliations
Contributions
GGNA, LHMF, MFD, JHLO, DD and JRP conceived the study and performed writing—review and editing; GGNA, LHMF, DD and JRP contributed to methodology; GGNA, LHMF and JRP were involved in formal analysis and investigation; GGNA and JRP performed writing—original draft preparation and funding acquisition; DD and JRP supervised the study. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Diego Demarco is the editor-in-chief of the Brazilian Journal of Botany, and this article was entirely handled by an Associate Editor.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40415_2021_768_MOESM4_ESM.tif
SM. 4 Reconstruction analysis of ancestral character state based on Parsimony criterion. a Sepal number. b Sepal union. c Corolla aestivation. d Petal number (TIF 28089 kb)
40415_2021_768_MOESM5_ESM.tif
SM. 5 Reconstruction analysis of ancestral character state based on Parsimony criterion. a Androecium. b Stamen number. c Filament appendages. d Filament union (TIF 28033 kb)
40415_2021_768_MOESM6_ESM.tif
SM. 6 Reconstruction analysis of ancestral character state based on Parsimony criterion. a Staminodes. b Pistillodes. c Carpel union. d Style-stigma proportion (TIF 28175 kb)
40415_2021_768_MOESM7_ESM.tif
SM. 7 Reconstruction analysis of ancestral character state based on Parsimony criterion. a Style union. b Stigma union. c Stigma type. d Stalk-like elongations (TIF 27959 kb)
40415_2021_768_MOESM8_ESM.tif
SM. 8 Reconstruction analysis of ancestral character state based on Parsimony criterion. a Intrastaminal disk. b Fruit type (TIF 13590 kb)
Rights and permissions
About this article
Cite this article
Alves, G.G.N., Fonseca, L.H.M., Devecchi, M.F. et al. What reproductive traits tell us about the evolution and diversification of the tree-of-heaven family, Simaroubaceae. Braz. J. Bot 45, 367–397 (2022). https://doi.org/10.1007/s40415-021-00768-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-021-00768-y