Abstract
Zanthoxylum species are credited with various uses in ethnomedicine due to their rich metabolite composition. In Kenya, these include management of cancer and microbial related ailments. However, there are limited reports showing how the bioactivity of Kenyan Zanthoxylum species is linked to their phytochemical profiles. This study therefore aimed at examining the chemical variation among five Zanthoxylum species found in Kenya (Z. chalybeum, Z. gilletii, Z. holtzianum, Z. paracanthum and Z. usambarense) using metabolomics approaches and the anti-oxidant and antimicrobial activities of these species. In a Folin–Ciocalteu test, the phenolic content of the stem bark extracts of these species were 73.083–145.272 mg TAE/g, while the alkaloids (in bromothymol blue chromogenic test) and flavonoids (in aluminium chloride test) were found to be 152.39–207.19 mg ME/g, and 109.416–186.413 mg CE/g, respectively. These extracts also exerted strong antioxidant activities in the 2,2-iphenyl-1-picrylhydrazyl (DPPH) and ferric ion reducing antioxidant power assays. In a broth dilution assay, the extract of the stem bark of Z. holtzianum ability showed the highest antimicrobial activity, followed by Z. chalybeum stem bark extract. The activities were positively correlated to both flavonoids and alkaloids concentrations, while the concentration of phenolics had weak negative correlation to antimicrobial activities. A chemometric analysis of the liquid-chromatography mass spectrometry profiles led to grouping of the species into three clusters. This study illustrates the variation in the bioactivity of Zanthoxylum species based on metabolite composition and justifies the wide usage of Zanthoxylum species in Kenyan traditional medicinal practices.
Graphical abstract

Similar content being viewed by others
Introduction
Secondary metabolites are known to affect biological systems by exhibiting major properties such as antiviral, antimicrobial, antifungal, antimalarial, analgesic, antiarrhythmic, antihypertensive, psychoactive, and tumor inhibition (Acamovic and Brooker 2005). Secondary plant metabolites profiles and their associated concentrations vary enormously between plant species of the same genus (Neugart et al. 2018). Among other factors known to influence this variation, genetic related factors are the most recognized to affect this the most. Significant correlation between phylogenetic difference and chemical diversity is known to exist and the biological activity in medicinally important plants has been reported to be reliant on both of these aspects (Rønsted et al. 2012). The relationship of genetic and chemical diversity within plant taxa can thus be used as a guide to point to those taxonomic groups that hold the greatest pharmacological potential (Lautie et al. 2020).
The genus Zanthoxylum Linn belongs to the family Rutaceae and is a large genus with approximately 250 species of aromatic prickly trees or shrubs distributed in warm temperate and subtropical regions globally. In Kenya, this genus is represented by seven species, five of which are distributed in various agroecological zones while two species are known to be rare. Data on phylogenetic analysis conducted on pantropical Zanthoxylum species did not capture majority of the Kenyan species (Appelhans et al. 2018). However, chemotaxonomy and classical taxonomy (Beentje et al. 1994; Kaigongi and Lukhoba 2021) delineated these species to three clades. Alkaloids, flavonoids, lignans, amides, sterols, and coumarins are commonly reported phytochemicals that are recognized to be predominant in this genus (Adesina 2005; Patiño et al. 2012; Tian et al. 2017; Kaigongi et al. 2020b). The ethnobotanical uses of this genus in Kenya suggest that its members possess antimicrobial and antioxidant properties. For example, a Zanthoxylum chalybeum leaf decoction is used to treat oedema in kwashiorkor and snake bites, whereas a root decoction is drunk for the treatment of chest pain and pneumonia. Decoctions made from bark of Z. chalybeum are drunk for the treatment of malaria, colds, coughs, dizziness, whilst the bark is also chewed for toothache (Kokwaro 2009; Kaigongi and Musila 2015). For Z. gilletii, the bark is chewed and the juice swallowed for the treatment of stomach ache (Kokwaro 2009) with decoction of young leaves being used to ease coughs (Nyunja et al. 2009). The young twigs of Z. usambarense, on the other hand, are used as chew sticks for dental hygiene while stem and root bark decoctions are commonly taken by the Maasai people as a cough mixture and to treat pneumonia (Maundu et al. 2001). The stem and root extracts of Z. paracanthum are used in the management of microbial and tumour related diseases (Kaigongi et al. 2020b). Despite the wide use of the genus Zanthoxylum in the Kenyan traditional medicine, only a few reports exist on phytochemical profiling and evaluation of antimicrobial and antioxidant activities of this group of plants (Buyinza 2012; Gaya et al. 2013; Kaigongi 2014a, b, 2020b; Okagu et al. 2021).
Metabolomics is an emerging field of science that aims at analysing potentially large numbers of complex secondary metabolite profiles, while permitting at the same time, the investigation of discrete metabolomic features across many profiles (Bino et al. 2004). Metabolomics approaches have been broadly applied in many fields for example; phytopharmaceutical manufacturing, medicinal plants quality control, toxicological survey, nutritional biochemistry and characterization of bioactive compounds in drug discovery (Yuliana et al. 2011, 2013; Cox et al. 2014; Chagas-Paula et al. 2015). Using a liquid chromatography-mass spectrometry (LC-MS) approach, we thus applied metabolomics to establish the relationship between chemical variation among five Zanthoxylum species in Kenya (Z. chalybeum, Z. gilletii, Z. holtzianum, Z. paracanthum and Z. usambarense) with antimicrobial and anti-oxidant activities. All other antimicrobial studies of Kenyan Zanthoxylum focused on single species (Buyinza 2012; Gaya et al. 2013; Özkan et al. 2013; Kaigongi et al. 2014b, 2020b) thus creating a need to determine the best Zanthoxylum species in terms of antimicrobial activity. By using a genus-wide exploration of the different species in Kenya, we could thus determine whether species that are closely related in the current accepted phylogenetic framework, also show similar chemotaxonomic relatedness, that is aligned with their bioactivities studied here-in. This paper consequently adds further scientific evidence to endorse the popularity of this medicinal plant genus as an antimicrobial agent and directly correlates both anti-oxidant and antimicrobial bioactivities to interspecies-based chemical variation.
Materials and methods
Collection of plant materials
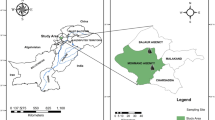
The stem bark of five Zanthoxylum investigated in this study (Table 1), was identified by Magrate Kaigongi (a botanist at Kenya Forestry Research Institute) and their voucher specimens deposited at the University of Nairobi Herbarium. Stem barks from three different individuals of each of the five species of approximately similar age were collected in April 2018 from four regions in Kenya (Fig. 1). The different species were collected from various localities because all species are known to occur naturally in different localities, except for Z. holtzianum and Z. paracanthum which occur together in the Kenyan coast. However, different parts of Zanthoxylum species contain effective compounds worthy investigating, for example the essential oils in leaves. We used stem barks in this study because they are the predominantly used Zanthoxylum part in Kenyan traditional medicine (Matu and Van Staden 2003; Kokwaro 2009; Kaigongi and Musila 2015; Odongo et al. 2017; Kaigongi et al. 2020b). The plant materials were washed, chopped into small pieces (≌ 2 cm), dried in the shade at room temperature and then finely ground in an aseptic manner.
Materials, reagents and equipment
Quercetin, and catechin standards, Folin Ciocalteu’s phenol reagent, tannic acid, sodium nitrite (NaNO2) analytical reagent, sodium carbonate (Na2CO3) powder, sodium hydroxide (NaOH) pellets, aluminium chloride hexahydrate, bromothymol blue chromogenic agent, chloroform omacilin, fluconazole, methanol, dichloromethane, dimethyl sulfoxide (DMSO), ascorbic acid standard, ferrous sulphate, ferrozine, trolox and 2,2-diphenyl-1-picrylhydrazyl (DPPH), dichloromethane (DCM) and methanol (MeOH) were obtained from Sigma Aldrich Co. (St Louis, MO, USA). Stigmasterol, acetonyldihydrochelerythrine, sesamin, arnottianamide, methoxycanthin-6-one, oxochelerythrine, and canthin-6-one standards were isolated and purified from Z. paracanthum reported by Kaigongi et al. (2020b). A Büchi Rotavapor R-200 rotary evaporator (Büchi Labortechnik, Flawil) was used to evaporate extracting solvents from plant extracts. A Waters Synapt G2 quadrupole time-of-flight mass spectrometer (Milford, MA, USA) linked to a Waters Acquity UPLC was used to perform liquid chromatography- mass spectroscopy (LC–MS) analysis. For all spectrophotometric analyses, a Helios Omega UV-spectrophotometer (Thermo Scientific technologies, Madison, USA) was used to read the absorbance in the determination of antioxidant activity. MassLynx™ version 4.1 software (Waters Corporation, Boston) was used to acquire and process metabolite spectra.
Samples extraction
Fifty grams of each ground material were separately exhaustively and sequentially extracted with CH2Cl2/CH3OH (1:1). The resultant extracts were filtered through Whatman filter paper 41 to remove debris. The filtrates were dried in vacuo using a rotary evaporator at 50 °C.
Determination of total phenolic content (TPC)
The total phenolic content was determined according to the Folin–Ciocalteu method described by Zonyane et al. (2020) with modifications. Each extract from the stem bark of five Zanthoxylum species was dissolved in 10% DMSO to give a concentration 0.5 mg/ml. An aliquot of the plant extract, 0.5 ml, was mixed with 4 ml of deionized water and 1 ml of Folin–Ciocalteu reagent. After 5 min, 3 ml of 10% (v/v) sodium carbonate was added to the previous mixture and filled to 10 ml with deionized water. The absorbances of the reaction products were measured using a spectrophotometer at 765 nm after 2 h of incubation at 30 °C. The total phenolic contents of the extracts were expressed as milligrams of tannic acid equivalents (TAE) per gram of extract from a calibration curve of tannic acid (0.01–0.06 mg/mL diluted in 50% methanol; A = 0.017 c (tannic acid)—0.3873, R2 = 0.9986). Each extract was determined in triplicate.
Determination of total alkaloids content (TAC)
An aliquot, 1 ml of 0.5 mg/ml of each of the five Zanthoxylum species extracts dissolved in 10% DMSO was mixed with 10 ml of a solution made from 50% bromothymol blue chromogenic agent (pH 7.0) and 50% chloroform (v/v) and vortexed for 2 min. The mixture was transferred into a separating funnel and left to sit for 2 h. The chloroform layer at the bottom of the funnel was collected, of which 4 ml was removed and 0.2 mg of anhydrous sodium sulphate was added to the removed portion and shaken to dehydrate the solution. After sitting for 10 min, the absorbance was measured at 414 nm using a spectrophotometer. The total alkaloid concentration (TAC) of the extract was calculated using a standard curve of matrine (0.01–0.06 mg/mL) and expressed as milligrams of matrine equivalents (ME) per gram of dry weight of extract (mg ME/g extract) (Gan et al. 2017). Each extract was determined in triplicate.
Determination of total flavonoid content (TFC)
The aluminium chloride colorimetric method was used to measure the total flavonoid content (TFC) in the extracts (Zonyane et al. 2020). An aliquot of 0.5 ml dispensed from 0.5 mg/ml of each of the five Zanthoxylum species was mixed with 1.25 ml of distilled water and 0.75 ml of 5% sodium nitrite. After 5 min, 0.15 ml of 10% aluminium chloride was added, and 0.5 ml of 1 M sodium hydroxide solution 6 min later. The solution was then adjusted with distilled water to a final volume of 3 ml, mixed thoroughly and incubated for 60 min at room temperature. The absorbance was determined using a spectrophotometer at 510 nm wavelength. The total flavonoid content of the extract was calculated using a standard curve of catechin (0.01–0.06 mg/mL diluted in 50% methanol; A = 0.0047 (catechin) + 0.7495, R2 = 0.9999) and expressed as milligrams of catechin equivalents (CE) per gram of dry weight of extract (mg CE/g extract). All the extracts were tested in triplicate.
Antioxidant activity
The antioxidant activity was determined through radical scavenging potential and ferric reducing power ability using the DPPH and FRAP assays, respectively (Zonyane et al. 2020).
Radical-scavenging ability
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was used to determine the radical-scavenging ability of each extract. The DPPH assay was carried out in triplicates using CH3OH: CH2Cl2 extracts of different concentrations (10, 100 and 1000 μg/ml) as described by Yuliana et al. (2011) except that the dilutions were done using 10% DMSO. Ascorbic acid in similar concentrations was used as the positive control. A blank containing 10% DMSO instead of the test sample or ascorbic acid was used as the negative control. The free radical scavenging activity (RSA), determined by the decolouration of the DPPH solution, was calculated according to the formula:
where Atest is the absorbance of the reaction mixture containing the positive control or extract, and Ablank is the absorbance of the blank test.
The antioxidant capacity of the extracts was expressed as IC50 values and compared to ascorbic acid.
Reducing power ability
The reducing power of extracts from the five species of Zanthoxylum was carried out using the ferric ion reducing antioxidant power (FRAP) assay. The FRAP solution was prepared using 5 ml of 300 mM acetate buffer, 0.5 ml of 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) and 0.5 ml of 20 mM ferric chloride. An aliquot, 2.8 ml of FRAP solution, was added to 0.2 ml of CH3OH: CH2Cl2 extracts at different concentrations (10, 100 or 1000 μg/ml). Following the same procedure, a blank test containing 10% of DMSO instead of extract was included, while trolox at 10 μg/ml served as the positive control under the same condition. The reaction mixtures were incubated in the dark for 30 min. The reduction of the Fe3+-TPTZ complex to a coloured Fe2+-TPTZ complex by the extract was monitored by measuring the absorbance at 593 nm wavelength in 15 s intervals over the course of 30 min at 37 °C using a Helios Omega UV-spectrophotometer (Thermo Scientific technologies, Madison, USA). The changes in absorbance values of test reaction mixtures from the blank test reading were considered as FRAP activity and then expressed as mM Trolox equivalent per gram of extract (mM TE/g extract) (Fawole et al. 2012).
Antimicrobial activity
Minimal inhibition concentration (MIC)
The fungal suspension was standardized according to the Clinical and Laboratory Standards Institute (Wayne 2002). The fungi were grown in Sabouraud agar for 72 h and standardized with sterile saline to a turbidity equivalent based on the 0.5 McFarland scale (≌ 106 CFU/ml). Bacteria were grown and standardized according to the protocol of the Wikler (2006). This was done by growing them in Mueller–Hinton agar for 24 h followed by standardizing them with sterile saline to a turbidity equivalent of the 0.5 McFarland scale (≌ 105 CFU/ml) before they were stored at 4 °C.
A broth dilution method was used to determine the minimal inhibition concentration according to the method reported by Kaigongi et al. (2020b) against the six microbes all obtained from the Centre for Microbiology Research (CMR) and the Kenya Medical Research Institute (KEMRI). The microbes were: Escherichia coli (ATCC 25,922), Staphylococcus aureus (ATCC 29,213), Bacillus cereus (ATCC 11,778), un-typed isolate of methicillin resistant Staphylococcus aureus (MRSA), Candida albicans (ATCC 10,231) and Aspergillus flavus (ATCC 26,914). The plant extracts were serially diluted 2-fold from 1000 to 0.011 g/ml in 96 microwell plates. Omacilin and fluconazole, which were both bought from Sigma-Aldrich (USA), were used as positive controls for the bacteria and fungi respectively. The lowest dilution with undetectable bacterial growth was recorded as the MIC. A lack of growth of the microorganisms was validated by the absence of turbidity after inoculating into agar. This was then followed by incubations for 24 and 72 h, for bacteria and fungi to grow, respectively (Valgas et al. 2007). Samples were tested in triplicate for every concentration.
Metabolite profiling
Sample preparation for metabolite profiling
Fifty mg of the sample was extracted with 2 ml methanol with vortexing and sonication for 30 min. Thereafter, samples were left for 16 h in the solvent at room temperature. To remove cell debris, samples were filtered and diluted 10-fold before LC–MS analysis. Extractions were performed in triplicate for statistical processing and all samples were randomized to eliminate changes in the instrument conditions over time. Standards (quercetin, catechin, stigmasterol, acetonyldihydrochelerythrine, sesamin, arnottianamide, methoxycanthin-6-one, oxochelerythrine, and canthin-6-one) were analytically weighed out and dissolved in dimethyl sulfoxide (DMSO) and diluted in methanol to a calibration series of 10, 20, 50, 100, 200 mg/L (Albrecht et al. 2012; Kaigongi et al. 2020a).
Liquid chromatography mass spectrometry (LC–MS) analysis
The LC–MS analysis was conducted as described by Albrecht et al. (2012). Briefly, a Waters Synapt G2 Quadrupole time-of-flight (QTOF) mass spectrometer (MS) connected to a Waters Acquity ultra-performance liquid chromatograph (UPLC) (Waters, Milford, MA, USA) was used for high-resolution UPLC-MS analysis. Electrospray ionization was used in positive and negative modes with a cone voltage of 15 V, desolvation temperature of 275 °C, desolvation gas at 650 L/h, and the rest of the MS settings optimized for best resolution and sensitivity. Data were acquired by scanning from m/z 150 to 1500 m/z in the mass spectroscopy engine (MSE), allowing two channels of MS data to be acquired: f1 at a low collision energy (4 V) and f2 using a ramped a collision energy (20–60 V) to simultaneously obtain fragmentation data. Separation was achieved on a Waters ethylene bridged hybrid (BEH) C18, 2.1 × 100 mm, 1.7 μm column held at 55 °C. An injection volume of 2 μL was used and the mobile phase consisted of 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid as solvent B. The gradient started at 100% solvent A for 0.5 min, followed by a linear gradient to 22% acetonitrile (solvent B) over 2.5 min, 44% solvent B over 4 min and finally to 100% solvent B over 5 min. The column was subjected to a 100% solvent B wash for an additional 2 min and then re-equilibrated over 1 min to yield a total run time of 15 min. A flow rate of 0.4 ml min −1 was utilized. Data was acquired and processed using Masslynx 4.1 (Waters, Milford, USA). Three technical replicates were analysed for each sample.
Data analysis
Using GraphPad Prism (version 8.4.6), antioxidant data was analysed through one-way analysis of variance (ANOVA) followed by a post hoc test using the Dunnett Test to determine presence of any significant difference between the activities of the samples with that of the positive controls (Musila et al. 2017). In all cases, the data was analysed for normality using the Shapiro–Wilk Test before application of the ANOVA. For antimicrobial data, the minimal inhibition concentrations were noted for each extract against the targeted microbe. These data were then used to obtain Pearson’s correlation values and a matrix generated using the same software reported by Zonyane et al. (2020).
Waters *.raw data was converted to *.abf format using Reifycs Abf Converter and then processed using MSDial v4.6 to generate a matrix of retention time, m/z and peak height intensity data. Peak alignments were exported to MSFinder 3.5 to facilitate compound annotation by comparison of MS2 spectra with those generated by in-silico fragmentation against a host of in-built database libraries. Tentative compound names and peak height intensities’ were then uploaded to MetaboAnalyst for multivariate analysis. Targetlynx was used to quantify metabolites against a cocktail of compounds purified from the plants (10–200 mg/L) (Kaigongi et al. 2020a).
Identification of metabolites
Data were processed using MSDIAL and MSFINDER (RIKEN Center for Sustainable Resource Science: Metabolome Informatics Research Team, Kanagawa, Japan). Formula prediction was performed by MSFinder using a mass tolerance of 10 ppm for both MS1 and MS2 data. Various databases were selected within MSFinder, namely HMDB, YMDB, PlantCyc, ChEBI, STOFF, NPA, NANPDB, COCONUT, KNApSAcK, Pubchem and UNPD. A total score of greater than 5 was used as the cut-off threshold. Fragmentation spectra were also manually confirmed using MassLynx with upload to Metfrag and MoNA in certain instances. In certain cases, where standards were available, the MSE fragmentation patterns of these standard compounds provided further validation of the tentative identifications (Kaigongi et al. 2020a).
Results and discussion
Phytochemical concentrations
The concentrations of the three phytochemicals evaluated (phenolics, alkaloids and flavonoids) differed significantly in the stem bark of five Zanthoxylum species (Fig. 2).
Metabolites concentrations in the five Kenyan Zanthoxylum species. Blue bars represent total alkaloid concentration (mg ME/g extract) total flavonoid concentration (mg CE/g extract) and total phenolic concentration (mg TAE/g extract) are represented by pink and green coloured bars, respectively. Values are expressed as Mean ± Standard error of mean (SEM), species with different letters (a–e) above bars of similar colour denote statistically significant differences in metabolite concentration while those with similar letters indicate insignificant differences (p < 0.05)
Production of phytochemicals in both qualitative and quantitative profiles is significantly influenced by different agronomic conditions (developmental stage, plants organs, fertilization and soil pH), climatic factors such as light intensity and water availability as well as genetics (Björkman et al. 2011; Vázquez-León et al. 2017) In this study, all the five species recorded high concentration of alkaloids followed by flavonoids while phenolics were least abundant in the test species, except in Z. gilletii. Z. holtizianum, a species growing along the Kenyan coast had the highest concentrations of alkaloids (207.19 mg ME/g extract) and flavonoids (186.41 107 mg CE/g extract). This was followed closely by Z. chalybeum, a cosmopolitan species in Kenya mainly in the drylands. The average total concentration of phenolics in the evaluated species was 68.023–145.272 mg TAE/g extract where Z. holtzianum recorded the highest concentration followed by Z. gilletii (134.16 mg TAE/g extract) a species growing in the highlands of Kenya. The high concentration of alkaloids in the genus Zanthoxylum has been reported elsewhere (Patiño et al. 2012) For example, a report by Marquez et al. (2005) showed that alkaloids are the most important class of compounds for this genus as they are found in almost all species with high concentrations in stem and root barks. A study conducted on Ugandan Z. chalybeum (within East African region) reported high content of phenolics than that of alkaloids in both the leaf and stem bark extracts that were extracted using methanol (Nantongo et al. 2018).
Antioxidant activity
Antioxidants operate through several mechanisms of action, including inhibition of free radical generation, enhancement of scavenging capacity against free radicals, and reducing power; thus, no single assay can accurately reflect all of the antioxidants in a mixed or complex system. It is therefore necessary to use at least two different methods to evaluate the antioxidant capacities of products (Gan et al. 2017). Here, DPPH for radical scavenging activity and FRAP for reducing power were used. The two were chosen based on their convenience, sensitivity, rapid, reproducibility, sample-polarity independent and ability to provide preliminary information for lead-finding of novel antioxidants (Koleva et al. 2002). The DPPH assay is based on hydrogen donating ability of the plant extract by reducing DPPH to DPPH-H indicated by the bleaching of a purple-coloured DPPH solution (Nunes et al. 2012). The FRAP assay measures the ability of an antioxidant to reduce ferric(III) to ferrous(II) in a redox-linked colourimetric reaction that involves single electron transfer (Benzie and Strain 1996).
In this study, some degree of antioxidant activity was observed in all the evaluated extracts, with a direct relationship between antioxidant activity and concentration of plants extracts. Although all plants do show antioxidant activity as phenolics occur ubiquitously in nature in plants, there is still value in considering those plants with high antioxidant power as they can be explored further as high antioxidant capacity is often implicated in other pharmacological activities such as anticancer action (Zonyane et al. 2020). All the Zanthoxylum species indicated a dose-dependent trend for antioxidant activity of the tested extracts. The RSA values were significantly higher in Z. holtizianum (93.72%), Z. chalybeum (90.70%), Z. usambarense (86.59%) and Z. paracanthum (82.73) (p < 0.05), while the lowest activity was exhibited by Z. gilletii (81.77%) (Table 2). The RSA of ascorbic acid (70.64%), used in this study as a positive control, was lower than all the plant extracts at 1000 μg/ml.
As shown in Fig. 3, the antioxidant capacities of the five Zanthoxylum species varied with the concentration of the sample that can scavenge 50% of DPPH free radical (IC50) for DPPH radical scavenging activity ranging from 5.1 to 14.92 μg/ml. The Ferric ion reducing antioxidant power results from the FRAP assay indicated average activity of 9.13–17.20 mM TE/g extract. In all the two antioxidant assays, DPPH and FRAP the highest antioxidant activity among the species was found in Z. holtzianum and Z chalybeum. Furthermore, Z. holtzianum had the highest antioxidant activity of all while Z. gilletii had the lowest activity. Results from both DPPH and FRAP assays in this study were consistent with each other and therefore able to conclusively show species that were more potent in their bioactivities. Secondary metabolites are often similar within members of a clade which translates to likeness in their bioactive properties (Wink 2015). Although little information exists on molecular phylogeny of the Kenyan Zanthoxylum species, morphological phylogenetics showed that Z. chalybeum and Z. holtzianum belonged to one phenetic group while Z. usambarense, Z. gilletii and Z. paracanthum belonged to a different group (Beentje et al. 1994; Kaigongi and Lukhoba 2021). The antioxidant activity in this study therefore correlates to species that occur in a similar phenetic group.
Antioxidant activity of dichloromethane/methanol extracts of five Zanthoxylum species in Kenya. A shows the average IC50 values generated from DPPH-1,1-diphenyl-2-picrylhydrazyl assay, B FRAP- Ferric ion reducing antioxidant power. Values for each assay are expressed as Mean ± Standard error of mean (SEM), Species with different letters (a–e) above average bars in the same graph are significantly different while those with similar letters are insignificantly different (p < 0.05)
Antimicrobial activity of the genus Zanthoxylum
Zanthoxylum species are popularly known to treat microbial related ailments in the Kenyan traditional medicine (Kokwaro 2009). There has, however, been no consistency and congruency in recorded data on antimicrobial activity of members of this genus in Kenya as most studies are conducted on single species. This triggered the interest to evaluate the possibility of variation in antimicrobial activity of extracts from different Zanthoxylum species in Kenya. Normally, quantitative determination through minimal inhibition concentration (MIC) is recommended for proper evaluation of the antimicrobial activity of a plant extract (Compean and Ynalvez 2014). In the present study, MIC values (Table 3) for all the extracts were in the range 0.97–1000 µg/ml indicating the antimicrobial potential of five Zanthoxylum species. It is worth noting that MIC values are indirectly proportional to antimicrobial potential of an extract and thus high MIC values indicate less activity and vice versa (Manandhar et al. 2019).
All the five Zanthoxylum species evaluated for antimicrobial activity showed different minimal inhibition concentrations against different microbes. Overall, Z. holtzianum recorded the best results in the inhibition of all the selected microbes followed by Z. chalybeum, Z. usambarense, Z. paracanthum while Z. gilletii recorded the least activity.
There was a consistent trend in the MIC values of the different species against the six microbes evaluated. For example, very high MIC values were required to curb the growth of MRSA followed by C. albicans and E. coli. Inhibition of S. aureus resulted in low MIC values followed by B. cereus and finally A. flavus. While this is the first report on the antimicrobial activity of stem bark extracts of Z. paracanthum and Z. holtzianum, this study corroborates with literature reports (He et al. 2002; Matu and Van Staden 2003) on the broad spectrum antimicrobial activity of the root and stem bark extracts of Z. usambarense. This report concurs with findings from Gaya et al. (2013) and Kaigongi et al. (2014b) on the antimicrobial activity of Z. gilletii and Z. chalybeum respectively. The high antimicrobial activity in both Z. holtzianum and Z. chalybeum could be attributed to their phylogeny as the two belong to a common clade (Beentje et al. 1994; Kaigongi and Lukhoba 2021).
Correlation of phytochemical content, antimicrobial and antioxidant activities
Studies relating antioxidant and antimicrobial activities to alkaloids and flavonoids have been reported (Karou et al. 2005; Kumar et al. 2008; Adamczak et al. 2020). There was a consistent trend connecting the antioxidant and antimicrobial activities of the tested specimens. Z. holtzianum and Z. chalybeum were superior in terms of the two bioactivities while Z. gilletii was less effective. A Pearson’s correlation matrix (Fig. 4) was used to reveal the correlation of the phytochemical content, antioxidant and antimicrobial activities at p = 0.05. The degree of antioxidant activity depends mainly on the ability of compounds providing protons to neutralize free radicals at the initial stage of oxidation or terminating the radical chain reaction (Wu et al. 2013). The correlation between phytochemicals and antioxidant activity was strongly positive for alkaloids and flavonoids recording r values as 0.913 (p = 0.031) and 0.861 (p = 0.043), respectively against FRAP and 0.717 (p = 0.049) and 0.909 (p = 0.033) correspondingly against percentage DPPH. A weak/negative correlation was recorded for phenolics and antioxidant activity with r values of 0.286 (p = 0.641) and − 0.230 (p = 0.71) against percentage DPPH and FRAP respectively. Additionally, phenolics were negatively correlated to antimicrobial activity (r = − 0.008, p = 0.990) while alkaloids and flavonoids recorded a strong correlation (r = 0.806, p = 0.047) for alkaloids and r = 0.855 and p = 0.045 for flavonoids. The correlation for antimicrobial and antioxidant was positively recorded as r = 0.775 and p = 0.048 for FRAP and r = 0.938 and p = 0.019 for percentage DPPH. A significant correlation was considered at p ≤ 0.05. This implies that flavonoids and alkaloids significantly influenced the bioactivities of the tested extracts. A correlation of antimicrobial and antioxidant activities to the chemical content of the Zanthoxylum species in this study showed that the higher the alkaloids and flavonoids content, present in the different species, the lower the MIC, IC50 and DPPH values and thus the higher the antimicrobial activity. This study is consistent with research findings by Gan et al. (2017) who observed negative correlation between the total alkaloids with the antioxidant IC50 values in Maca plants. On the other hand, Farasat et al. (2014) and Kimondo et al. (2019) showed that flavonoid content caused low IC50 values, and hence the higher the antioxidant activity per gram of extract.
Correlation matrix based on Pearson’s coefficient values showing relationship of phytochemicals, antioxidant and antimicrobial activities. Significant correlation was considered at p ≤ 0.05. Colour intensities are directly proportional to correlation coefficients. Red and purple colours show strongest and weakest correlations respectively
Flavonoids have been shown to be highly effective scavengers of the most oxidizing molecules implicated in several diseases (Nithya et al. 2016). Reports on antioxidant activity of flavonoids indicate their ability on free radical scavenging activity, chelation of transition metals, quenching of singlet oxygen, and inhibition of enzymes (Cos et al. 2000). According to Heim et al. (2002) and Xie et al. (2015), the antioxidant and antimicrobial activities of flavonoids depend strongly on the number and position of hydroxyl groups in the molecule. Dihydroxylated B-ring (catechol structure), presence of 4-oxo function and unsaturation in the C-ring are also presumed to increase the antioxidant capacity. Alkaloids have a wide range of pharmacological activities including anticancer, anti-inflammatory, antidiabetic and antibacterial activities, and hence, are responsible for many healing properties in natural medicine (Fattorusso and Taglialatela-Scafati 2007). While a few reports exist on the alkaloids-antioxidant and antimicrobial structure relationship, Zhao et al. (2006) associated the high antioxidant activity of alkaloids to the nitrogen lone pair electron as well as the number and position of phenolic hydroxyl in their molecular structure.
Phenolics are very important in plants as they help in pigmentation, growth, reproduction, structural integrity and scaffolding support (Lattanzio et al. 2008). The weak correlation of phenolics to both antimicrobial and antioxidant potential in this study is contrary to the reports made by Rumbaoa et al. (2009) and Tungmunnithum et al. (2018) who found strong positive correlations between phenolic content and DPPH. These chemicals possess different bioactivities such as anticancer, antimicrobial, and antioxidant actions (Soobrattee et al. 2005; Ambriz-Pérez et al. 2016; Zonyane et al. 2020). However, phenolics are reported to possess strong antimicrobial activity (Jawhari et al. 2021), the weak correlation of these chemicals to antimicrobial activity recorded in this study is similar to findings by Osman et al. (2020) who recorded weak correlation between antimicrobial activity and phenolics in ten different Egyptian plants. Phenolics are polar compounds and thus the low levels recorded here could be attributed to the extracting solvents, a mixture of dichloromethane and methanol (1:1) (Şensoy et al. 2006; Rocchetti et al. 2020).
Biochemometric analysis
The isolation of bioactive compounds from natural sources is a key step in drug discovery and development, however, this procedure is usually expensive and difficult due to the complexity and the low concentration of some of the metabolites in the extracts. Thus, targeted identification of potential compounds is becoming more popular to reduce bottlenecks in natural products research (Plazas et al. 2019). Unsupervised metabolomic profiling of the data obtained from the LC–MS analysis of five extracts of Zanthoxylum produced a total of 3000 features (RT; m/z) in both positive and negative modes. Of these, 52 compounds were tentatively identified in positive mode (Table 4) and their spectra deposited to ftp://massive.ucsd.edu/MSV000088044/. Based on the PCA plot combining both the activities and the chemicals (Fig. 5), the five Zanthoxylum species stem bark extracts were grouped into three clusters. Zanthoxylum gilletii and Z. usambarense formed cluster 1 (green line) irrespective of them being from distinct locations. On the other hand, all extracts from both biological and technical replicates of Z. paracanthum were grouped in a totally separate cluster (red line), pointing out a chemical variability of this species compared to the others studied. Zanthoxylum holtizianum and Z. chalybeum formed cluster 2 (blue line). Members of a single cluster implied a similarity in terms of chemical composition while differences in clustering was brought about by varied phytochemicals being present. The reported antioxidant and antimicrobial activities of the tested species was likely due to the chemical composition of these plants as the members of different clusters showed similarity in terms of these bioactivities (Yuliana et al. 2011; Amenu 2014).
Some of the identified compounds have been reported to exhibit high antioxidant and antimicrobial activities. It is possible that the high bioactivities recorded in the studied Zanthoxylum species is due to synergistic effect from these compounds as claimed by Amenu (2014). The varied structure activity relationship of different compounds in an extract attributes to high bioactivity of the extract (Zhao et al. 2006; Fattorusso and Taglialatela-Scafati 2007; Tungmunnithum et al. 2018; Plazas et al. 2019). It is arguable that Z. holtizianum and Z. chalybeum had the best activities due to high relative abundance of both alkaloids and flavonoids compared to other species. Some of these chemicals, which were detected at higher relative abundances (Fig. 6) in the two species, are known to possess high antimicrobial and antioxidant activities including: canthin-6-one, chelerythrine, dihydrochelerythrine, arnottianamide, fagaronine, catechin, psoralen, chlorogenic acid, kaempferol 3-O-rhamnoside and quercetin 3-O-rhamnoside (Şensoy et al. 2006; Plazas et al. 2019; Kaigongi et al. 2020b; Rocchetti et al. 2020). It is apparent that metabolomics has been shown to be a potential facilitator in the discovery of natural products as outstanding sources for novel leads (Soobrattee et al. 2005; Rumbaoa et al. 2009; Ambriz-Pérez et al. 2016). This made it possible for profiling of metabolites that were shown in the studied plants for the first time in this study as listed in Table 4.
Conclusion
The incorporation of metabolomics-based tools into natural product screening helped us to discover novel chemical scaffolds through metabolite-mining methods. All the species studied here exhibited antioxidant and antimicrobial potential. The stem bark extracts of Z. holtzianum and Z. chalybeum had the highest levels of alkaloids and flavonoids. These were correlated to the high antioxidant and antibacterial activities recorded in the two species. However, phenolics are well known as antioxidant molecules, but, their content in the Zanthoxylum species analysed were low and showed weak correlations with the two tested bioassays. Based on these results, there is need to isolate the specific alkaloids and flavonoid compounds that are correlated with the antioxidant activity of Zanthoxylum as well as to follow with an evaluation of synergistic interactions of these chemicals. The antioxidant activity of leaf extracts of this group of plants needs to be investigated as leaves are sustainable medicinal plant materials compared to stem and root barks. This study justifies the use of Zanthoxylum species in the Kenyan traditional medicine system and could be exploited as a potential source of natural antimicrobial and antioxidant agents. Stem bark extracts of Z. holtzianum and Z. chalybeum are reported as superior species in terms of antioxidant and antimicrobial properties compared to other Kenyan Zanthoxylum species.
Data availability
Not applicable.
References
Acamovic T, Brooker JD (2005) Biochemistry of plant secondary metabolites and their effects in animals. Proc Nutr Soc 64:403–412
Adamczak A, Ożarowski M, Karpiński TM (2020) Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J Clin Med 9:109
Adesina SK (2005) The Nigerian Zanthoxylum; chemical and biological values. Afr J Tradit Complement Altern Med 2:282–301
Albrecht CF (2012) LC–MS-based metabolomics assists with quality assessment and traceability of wild and cultivated plants of Sutherlandia frutescens (Fabaceae ). S Afr J Bot 82:33–45. https://doi.org/10.1016/j.sajb.2012.07.018
Ambriz-Pérez DL, Leyva-López N, Gutierrez-Grijalva EP, Heredia JB (2016) Phenolic compounds: natural alternative in inflammation treatment. Rev Cogent Food Agric 2:1131412
Amenu D (2014) Antimicrobial activity of medicinal plant extracts and their synergistic effect on some selected pathogens. Am J Ethnomed 1:18–29
Appelhans MS, Reichelt N, Groppo M, Paetzold C, Wen J (2018) Phylogeny and biogeography of the pantropical genus Zanthoxylum and its closest relatives in the proto-Rutaceae group (Rutaceae). Mol Phylogenet Evol 126:31–44
Beentje H, Adamson J, Bhanderi D (1994) Kenya trees, shrubs, and lianas. National Museums of Kenya
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Bino RJ, Hall RD, Fiehn O, Kopka J, Saito K, Draper J, Nikolau BJ, Mendes P, Roessner-Tunali U, Beale MH (2004) Potential of metabolomics as a functional genomics tool. Trends Plant Sci 9:418–425
Björkman M, Klingen I, Birch ANE, Bones AM, Bruce TJA, Johansen TJ, Meadow R, Mølmann J, Seljåsen R, Smart LE (2011) Phytochemicals of Brassicaceae in plant protection and human health: influences of climate, environment and agronomic practice. Phytochemistry 72:538–556
Buyinza D (2012) Phytochemical investigation of Zanthoxylum holstzianum for antimicrobial activity. MSc thesis, University of Nairobi
Chagas-Paula DA, Oliveira TB, Zhang T, Edrada-Ebel R, Da Costa FB (2015) Prediction of anti-inflammatory plants and discovery of their biomarkers by machine learning algorithms and metabolomic studies. Planta Med 81:450–458
Compean KL, Ynalvez RA (2014) Antimicrobial activity of plant secondary metabolites: a review. Res J Med Plants 8:204–213
Cos P, Calomme M, Pieters L, Vlietinck AJ, Vanden BD (2000) Structure-activity relationship of flavonoids as antioxidant and pro-oxidant compounds. Stud Nat Prod Chem 22:307–341
Cox DG, Oh J, Keasling A, Colson KL, Hamann MT (2014) The utility of metabolomics in natural product and biomarker characterization. Biochim Biophys Acta (BBA) Gen Stud 1840:3460–3474
Farasat M, Khavari-Nejad R-A, Nabavi SMB, Namjooyan F (2014) Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf. Iran J Pharm Res IJPR 13:163
Fattorusso E, Taglialatela-Scafati O (2007) Modern alkaloids: structure, isolation, synthesis, and biology. John Wiley & Sons
Fawole OA, Makunga NP, Opara UL (2012) Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement Altern Med 12:1–11
Gan J, Feng Y, He Z, Li X, Zhang H (2017) Correlations between antioxidant activity and alkaloids and phenols of maca (Lepidium meyenii). J Food Qual 2017:1−10
Gaya CH, Kawaka JF, Muchugi A, Ngeranwa JJ (2013) Variation of alkaloids in the Kenyan Zanthoxylum gilletii (De Wild Waterman). Afr J Plant Sci 7:438–444
He W, Van PL, De KN, Verbruggen L, Anthonissen K, Van der Flaas M, Bosselaers J, Mathenge SG, Mudida FP (2002) Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother Res 16:66–70
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584
Jawhari FZ, Moussaoui AE, Bourhia M, Imtara H, Saghrouchni H, Ammor K, Bari A (2021) Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: correlation between total phenolic and flavonoid contents with antioxidant and antimicrobial activities of chemically characterized extracts. Plants 10:149
Kaigongi MM (2014a) Antimicrobial activity, toxicity and phytochemical analysis of four medicinal plants traditionally used in Msambweni district, Kenya. MSc Diss, University of Nairobi
Kaigongi MM, Dossaji SF, Nguta JM, Lukhoba CW, Musila FM (2014b) Antimicrobial activity, toxicity and phytochemical screening of four medicinal plants traditionally used in Msambweni district, Kenya. J Biol Agric Healthc 4:6−12
Kaigongi MM, Lukhoba CW, Taylor M, Yenesew A, Makunga NP (2020a) LC-MS-based metabolomics for the chemosystematics of Kenyan Dodonaea viscosa Jacq (Sapindaceae) Populations. Molecules 25:4130
Kaigongi MM, Lukhoba CW, Yaouba S, Makunga NP, Githiomi J, Yenesew A (2020b) In vitro antimicrobial and antiproliferative activities of the root bark extract and isolated chemical constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae). Plants 9:920
Kaigongi MM, Lukhoba CW (2021) The chemosystematics of the genus Zanthoxylum L. (Rutaceae) in Kenya. Biochem Syst Ecol 98:104319
Kaigongi M, Musila F (2015) Ethnobotanical study of medicinal plants used by Tharaka people of Kenya. Int J Ethnobiol Ethnomed 1:1–8
Karou D, Savadogo A, Canini A, Yameogo S, Montesano C, Simpore J, Colizzi V, Traore AS (2005) Antibacterial activity of alkaloids from Sida acuta. Afr J Biotechnol 4:195–200
Kimondo J, Mutai P, Njogu P, Kimwele C (2019) Evaluation of the antioxidant activity of nine plants used medicinally by the Ilkisonko Maasai community of Kenya. Free Radic Antioxi 9:29–34
Kokwaro JO (2009) Medicinal plants of east Africa. University of Nairobi press
Koleva II, Van Beek TA, Linssen JPH, de Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal Int J Plant Chem Biochem Tech 13:8–17
Kumar PS, Sucheta S, Deepa VS, Selvamani P, Latha S (2008) Antioxidant activity in some selected Indian medicinal plants. Afr J Biotechnol 7:1826–1828
Lattanzio V, Kroon PA, Quideau S, Treutter D (2008) Plant phenolics—secondary metabolites with diverse functions. Recent Adv Polyphen Res 1:1–35
Lautie E, Russo O, Ducrot P, Boutin JA (2020) Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol 11:397
Manandhar S, Luitel S, Dahal RK (2019) In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J Trop Med 2019:1895340
Marquez L, Aguero J, Hernandez I, Garrido G, Martinez I, Dieguez R, Prieto S, Rivas Y, Molina-Torres J, Curini M (2005) Anti-inflammatory evaluation and phytochemical characterization of some plants of the Zanthoxylum genus. Acta Farm Bonaer 24:325
Matu EN, Van Staden J (2003) Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. J Ethnopharmacol 87:35–41
Maundu P, Berger D, Ole Saitabau C, Nasieku J, Kipelian M, Mathenge S, Morimoto Y, Höft R (2001) Ethnobotany of the Loita Maasai. People Plants Work Pap 8:1–34
Musila FM, Nguta JM, Lukhoba CW, Dossaji SF (2017) Antibacterial and antifungal activities of 10 Kenyan Plectranthus species in the Coleus clade. J Pharm Res 11:1003–1015
Nantongo JS, Odoi JB, Abigaba G, Gwali S (2018) Variability of phenolic and alkaloid content in different plant parts of Carissa edulis Vahl and Zanthoxylum chalybeum Engl. BMC Res Notes 11:1–5
Neugart S, Baldermann S, Hanschen FS, Klopsch R, Wiesner-Reinhold M, Schreiner M (2018) The intrinsic quality of brassicaceous vegetables: how secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci Hortic (amst) 233:460–478
Nithya TG, Jayanthi J, Ragunathan MG (2016) Antioxidant activity, total phenol, flavonoid, alkaloid, tannin, and saponin contents of leaf extracts of Salvinia molesta DS Mitchell (1972). Asian J Pharm Clin Res 9:200–203
Nunes XP, Silva FS, Almeida JRG da S, Barbosa Filho JM, de Lima JT, de Araújo Ribeiro LA, Júnior LJQ (2012) Biological oxidations and antioxidant activity of natural products. INTECH Open Access Publisher, New York
Nyunja ARO, Onyango JC, Erwin B (2009) The Kakamega forest medicinal plant resources and their utilization by the adjacent Luhya community. Int J Trop Med 4:82–90
Odongo E, Mungai N, Mutai P, Karumi E, Mwangi J, Kimondo J, Omale J, Simiyu J (2017) Antioxidant and anti-inflammatory activities of selected medicinal plants from western Kenya. Afr J Pharmacol Ther 6:178–182
Okagu IU, Ndefo JC, Aham EC, Udenigwe CC (2021) Zanthoxylum species: a comprehensive review of traditional uses, phytochemistry. Pharmacol Nutraceutical Appl Mol 26:4023
Osman MA, Mahmoud GI, Shoman SS (2020) Correlation between total phenols content, antioxidant power and cytotoxicity. Biointerface Res Appl Chem 11:10640–10653
Özkan M, Mutiso PBC, Nahar L, Liu P, Brown S, Wang W, Sarker SD (2013) Zanthoxylum usambarense (Engl.) Kokwaro (Rutaceae) extracts inhibit the growth of the breast cancer cell lines MDA-MB-231 and MCF-7, but not the brain tumour cell line U251 in vitro. Phytother Res 27:787–790
Patiño LOJ, Prieto RJA, Cuca SLE (2012) Zanthoxylum genus as potential source of bioactive compounds. Bioact Compd Phytomed 10:185–218
Plazas E, Casoti R, Murillo MA, Da Costa FB, Cuca LE (2019) Metabolomic profiling of Zanthoxylum species: identification of anti-cholinesterase alkaloids candidates. Phytochemistry 168:112128
Rocchetti G, Pagnossa JP, Blasi F, Cossignani L, Piccoli RH, Zengin G, Montesano D, Cocconcelli PS, Lucini L (2020) Phenolic profiling and in vitro bioactivity of Moringa oleifera leaves as affected by different extraction solvents. Food Res Int 127:108712
Rønsted N, Symonds MRE, Birkholm T, Christensen SB, Meerow AW, Molander M, Mølgaard P, Petersen G, Rasmussen N, Van Staden J (2012) Can phylogeny predict chemical diversity and potential medicinal activity of plants? A case study of Amaryllidaceae. BMC Evol Biol 12:1–12
Rumbaoa RGO, Cornago DF, Geronimo IM (2009) Phenolic content and antioxidant capacity of Philippine sweet potato (Ipomoea batatas) varieties. Food Chem 113:1133–1138
Şensoy Í, Rosen RT, Ho C-T, Karwe MV (2006) Effect of processing on buckwheat phenolics and antioxidant activity. Food Chem 99:388–393
Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T (2005) Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res Mol Mech Mutagen 579:200–213
Tian Y, Zhang C, Guo M (2017) Comparative study on alkaloids and their anti-proliferative activities from three Zanthoxylum species. BMC Complement Altern Med 17:1–16
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5:93
Valgas C, de Souza SM, Smânia EFA, Smânia A Jr (2007) Screening methods to determine antibacterial activity of natural products. Braz J Microbiol 38:369–380
Vázquez-León LA, Páramo-Calderón DE, Robles-Olvera VJ, Valdés-Rodríguez OA, Pérez-Vázquez A, García-Alvarado MA, Rodríguez-Jimenes GC (2017) Variation in bioactive compounds and antiradical activity of Moringa oleifera leaves: influence of climatic factors, tree age, and soil parameters. Eur Food Res Technol 243:1593–1608
Wayne PA (2002) Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI Doc M27-A2
Wikler MA (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. CLSI 26:M7-A7
Wink M (2015) Modes of action of herbal medicines and plant secondary metabolites. Medicines 2:251–286. https://doi.org/10.3390/medicines2030251
Wu AZ, Lin CZ, Zhu CC (2013) Progress in structure-activity relationship of phenylethanoid glycosides. Nat Prod Res Dev 25:862–865
Xie Y, Yang W, Tang F, Chen X, Ren L (2015) Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem 22:132–149
Yuliana ND, Khatib A, Choi YH, Verpoorte R (2011) Metabolomics for bioactivity assessment of natural products. Phytother Res 25:157–169
Yuliana ND, Jahangir M, Verpoorte R, Choi YH (2013) Metabolomics for the rapid dereplication of bioactive compounds from natural sources. Phytochem Rev 12:293–304
Zhao Q, Zhao Y, Wang K (2006) Antinociceptive and free radical scavenging activities of alkaloids isolated from Lindera angustifolia Chen. J Ethnopharmacol 106:408–413
Zonyane S, Fawole OA, La Grange C, Stander MA, Opara UL, Makunga NP (2020) The implication of chemotypic variation on the anti-oxidant and anti-cancer activities of Sutherlandia frutescens (L.) R. Br. (Fabaceae) from different geographic locations. Antioxidants 9:152
Funding
This research was partly funded by National Research Foundation (NRF) of South Africa as an award to Nokwanda Makunga and the N.R.F. of Kenya as an award to Catherine Lukhoba (under the Kenya-South Africa bilateral research program; Grant Number 105810) and the Kenya Forestry Research Institute (KEFRI) made contributions through Joseph Githiomi.
Author information
Authors and Affiliations
Contributions
The following authors contributed to this study: Conceptualization, (MK, CL, AY and NM); Methodology, (MK, MT and RM); Data analysis, (MK, MT and FM); Resources, (CL, AY, JG and NM); Writing-original draft preparation, (MK) Writing—review and editing (NM), Supervision, (CL and AY). All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies involving animals performed by any of the authors. This article does not contain any studies involving human participants performed by any of the authors.
Conflict of interest
Magrate M. Kaigongi has no conflict of interest. Catherine W. Lukhoba has no conflict of interest. Fredrick M. Musila has no conflict of interest. Malcolm Taylor has no conflict of interest. Regina W. Mbugua has no conflict of interest. Joseph Githiomi has no conflict of interest. Abiy Yenesew has no conflict of interest. Nokwanda P. Makunga has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaigongi, M.M., Lukhoba, C.W., Musila, F.M. et al. A versatile untargeted metabolomics-driven technology for rapid phytochemical profiling of stem barks of Zanthoxylum species with antioxidant and antimicrobial activities. ADV TRADIT MED (ADTM) 23, 929–945 (2023). https://doi.org/10.1007/s13596-022-00676-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-022-00676-w