Abstract
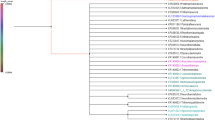
The genus Oxygyne comprises three species disjunctly distributed in Africa and Japan and is the least examined genus of the Burmanniaceae due to the scarcity of living material. We obtained living samples of Oxygyne shinzatoi and examined the phylogenetic position of this species on the basis on the 18S rDNA sequence. Oxygne shinzatoi was consistently found to belong to the monophyletic group of tribe Thismieae, but its position in the tribe differed depending on the criteria applied (maximum parsimony, maximum likelihood, Bayesian inference). Distance analysis from the most recent common ancestor indicated that O. shinzatoi had the lowest substitution rate among the species of tribe Thismieae. Combined with recent knowledge of basic chromosome numbers and substitution rate characteristics, O. shinzatoi can be considered to be one of the basal taxon of tribe Thismieae.



Similar content being viewed by others
References
Abe C, Akasawa Y (1989) A new species of Oxygyne (Burmanniaceae) found in Shikoku, Japan. J Jpn Bot 64:161–164
Bousalem M, Arnau G, Houch I, Arnolin R, Viader V, Santoni S, David J (2006) Microsatellite segregation analysis and cytogenetic evidence for tetrasomic inheritance in the American yam Dioscorea trifida and a new basic chromosome number in the Dioscoreae. Theor Appl Genet 113:439–451
Caddick LR, Rudall PJ, Wilkin P, Hedderson AJ, Chase MW (2002) Phylogenetics of Dioscoreales based on combined analyses of morphological and molecular data. Bot J Linn Soc 138:123–144
Chatterjee A, Ghosh S, Roy SC (1989) A cytological survey of eastern Himalayan plants III. Cell Chromosome Res 12:22–29
Chin H, Chang M, Ling P, Ting C, Dou F (1985) A cytological study on Chinese Dioscorea L. – the chromosome numbers and their relation to the origin and evolution of the genus. Acta Phytotax Sin 23:11–18
Essad S (1984) Variation géographique des nombres chromosomiques de base et polyploïdie dans le genre Dioscorea a propos du dénombrement des espèces transversa Brown, pilosiuscula Bert et trifida. Agronomie 4:611–617
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Hatusima S (1976) Two new species of Burmanniaceae from Japan. J Geobot 24:2–6
Huelsenbeck JP, Ronquist F (2001) MRBAYES. Bayesian inference of phylogeny. Bioinformatics 17:754–755
Imhof S (1999) Anatomy and mycotrophy of the achlorophyllous Afrothismia winkleri. New Phytol 144:533–540
Kubitzki K (1998) Taccaceae. In: Kubitzki K (ed) The families and genera of vascular plants. III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae). Springer, Berlin, pp 425–428
Maas-van de Kamer H (1998) Burmanniaceae. In: Kubitzki K (ed) The families and genera of vascular plants. III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae). Springer, Berlin, pp 154–164
Maddison WP (1991) The discovery and importance of multiple islands of most-parsimonious trees. Syst Zool 40:315–328
Merckx V, Schols P, Maas-van de Kamer H, Maas P, Huysmans S, Smets E (2006) Phylogeny and evolution of Burmanniaceae (Dioscoreales) based on nuclear and mitochondrial data. Am J Bot 93:1684–1698
Nickrent DL, Duff RJ, Colwell AE, Wolfe AD, Young ND, Steiner KE, Depamphilis CW (1998) Molecular phylogenetic and evolutionary studies of parasitic plants. In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematics of plants II, DNA sequencing. Kluwer, New York, pp 211–241
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Schlechter R (1906) Burmanniaceae africanae. Bot Jahrb Syst 37:140–141
Segarra-Moragues JG, Catalán P (2003) Life history variation between species of the relictual genus Borderea (Dioscoreaceae): phylogeography, genetic diversity, and population genetic structure assessed by RAPD markers. Biol J Linn Soc 80:483–498
Swofford DL (1998) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer, Sunderland
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Tsukaya H, Yokota M, Okada H (2007) Chromosomal characteristics of Oxygyne shinzatoi (Hatus.) C. Abe et Akasawa (Burmanniaceae), and its phylogenetic significance. Acta Phytotax Geobot 58:100–106
White TJ, Bruns TD, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M et al. (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Wilcox TP, Garcia de Leon FJ, Hendrickson DA, Hillis DM (2004) Convergence among cave catfishes: long-branch attraction and a Bayesian relative rates test. Mol Phylogenet Evol 31:1101–1113
Woodward CL, Berry PE, Maas-van de Kamer H, Swing K (2007) Tiputinia foetida, a new mycoheterotrophic genus of Thismiaceae from Amazonian Ecuador, and a likely case of deceit pollination. Taxon 56:157–162
Acknowledgments
The authors are grateful to S. Higa, K. Shinjo, and K. Ishii for assistance in collecting the materials. This study was supported in part by Grants-in-Aid for Scientific Research (C) (no. 17570083) to M. Y. and the twenty-first century COE program of the University of the Ryukyus.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10265-008-0175-7
Rights and permissions
About this article
Cite this article
Yokoyama, J., Koizumi, Y., Yokota, M. et al. Phylogenetic position of Oxygyne shinzatoi (Burmanniaceae) inferred from 18S rDNA sequences. J Plant Res 121, 27–32 (2008). https://doi.org/10.1007/s10265-007-0136-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-007-0136-6