Overview
Neurothekeoma is a benign, predominantly cutaneous neoplasm that was initially described by Harkin and Reed in 1969 by the name nerve sheath myxoma. [1] In 1980, Gallager and Helwig termed the lesion neurothekeoma (Greek: theke, sheath) to connote both the histologic nested (theque) appearance and the purported nerve sheath differentiation. [2] To date, there remains inconsistency in the literature regarding whether nerve sheath myxoma should be included in the morphologic spectrum of neurothekeoma, as cellular neurothekeoma stains for fibrohistiocytic markers and PRAME while nerve sheath myxoma stains for neural markers. [3, 4] Some hybrid forms have been reported. [5]
Based on the amount of myxoid matrix, neurothekeoma is histologically divided into myxoid, intermediate, and cellular types. It is generally agreed that the myxoid variant of neurothekeoma is of neural origin. The cellular variant, however, is of questionable origin, as it lacks both consistent neural immunoreactivity and ultrastructural evidence of neural differentiation.
The image below depicts the histologic appearance of a myxoid neurothekeoma.
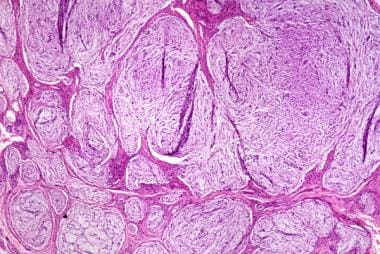 This myxoid neurothekeoma displays a lobulated, nonencapsulated but well-circumscribed proliferation of spindled and epithelioid cells embedded within a myxoid stroma.
This myxoid neurothekeoma displays a lobulated, nonencapsulated but well-circumscribed proliferation of spindled and epithelioid cells embedded within a myxoid stroma.
Neurothekeomas are generally seen to arise in the first 3 decades of life, with a female preponderance. In their study of 178 tumors, Fetsch and coworkers reported patient ages ranging from 20 months to 85 years, with a mean age of 21 years. [3] The male-to-female ratio was approximately 1:2. Similarly, in a study of 133 cases of cellular neurothekeoma, Hornick and Fletcher reported patient ages ranging from 1 to 65 years, with a mean age of 25 years. [6] The male-to-female ratio was 1:1.8.
Etiology
Limited ultrastructural studies have revealed few specific features in neurothekeoma. The myxoid variant is assumed to be schwannian based on ultrastructural and immunohistochemical studies. [7, 8]
Debate remains as to the etiology of cellular neurothekeoma. Some authors argue the fact that cellular neurothekeomas can show myxoid areas similar to classic myxoid neurothekeoma means they must have similar neural differentiation. [9] Other authors have theorized that cellular neurothekeoma may have myofibroblastic or fibrohistiocytic differentiation. [6] It has also been proposed that cellular neurothekeoma represents a variant of plexiform fibrohistiocytic tumor, atypical fibroxanthoma or cellular dermatofibroma, but both immunohistochemical and ultrastructural differences suggest neurothekeoma may be a unique entity. [10, 11, 12, 13]
Location and Clinical Features
Neurothekeomas most commonly occur on the head and neck. Specifically, the nose and scalp are the most frequent sites of involvement. These regions are followed by the orbital region, cheeks, and chin. [3] The upper and lower extremities and the trunk are other commonly reported sites of involvement. Cases of neurothekeomas occurring in the oral mucosa, paranasal sinuses, and eyelids have also been reported. [14] On cutaneous examination, neurothekeomas appear as asymptomatic to mildly tender, flesh-colored solitary nodules. An associated component of overlying erythema may be present. The nodules are most commonly less than 2 cm in diameter.
Neurothekeomas may be mistaken clinically for epidermal inclusion cysts, intradermal nevi, lipomas, pilomatrixomas, or dermatofibromas.
Gross and Microscopic Features
On gross examination, neurothekeomas appear as nondistinctive dermal tumors, most commonly measuring 0.5 to 1 cm in dimension.
Histologically, neurothekeomas consist of spindled and epithelioid cells with abundant eosinophilic, finely granular cytoplasm. The cells are situated in multiple, closely spaced nodules of varying sizes within the dermis.
Myxoid subtype
The myxoid subtype of neurothekeoma is characterized by a lobulated, nonencapsulated but well-circumscribed proliferation of spindled and epithelioid cells in varying proportions (see the first image below). The cells are embedded within a myxoid stroma. Nests are separated by varying amounts of collagen. Due to the large amount of myxoid stroma separating the cells, the tumor cells are said to display a random growth pattern (see the second image below). The histologic features of myxoid neurothekeoma are distinguishing enough that its diagnosis is usually not problematic. [15, 16]
 This myxoid neurothekeoma displays a lobulated, nonencapsulated but well-circumscribed proliferation of spindled and epithelioid cells embedded within a myxoid stroma.
This myxoid neurothekeoma displays a lobulated, nonencapsulated but well-circumscribed proliferation of spindled and epithelioid cells embedded within a myxoid stroma.
 Higher power view of the myxoid neurothekeoma from the previous image demonstrating the random growth pattern.
Higher power view of the myxoid neurothekeoma from the previous image demonstrating the random growth pattern.
Cellular subtype
The cellular subtype is a poorly circumscribed tumor involving the superficial dermis with possible extension into the superficial subcutis. Infiltration into fat and entrapment of skeletal muscle can be seen. The tumor consists of nests and fascicles of epithelioid cells with vesicular nuclei containing scant myxoid stroma (see the following image). Due to the fact that the myxoid element determines the size of tumor nodules, the nodules of cellular neurothekeoma are smaller than those of the myxoid subtype. [17] Although the myxoid variant of neurothekeoma is hypocellular, the cellular subtype is hypercellular. Sclerotic collagen is seen more markedly in cellular than myxoid tumors. Osteoclastic giant cells are seen in a minority of cases.
 Neurothekeoma Pathology. Cellular neurothekeoma consists of nests and fascicles of epithelioid cells with vesicular nuclei that contain scant myxoid stroma.
Neurothekeoma Pathology. Cellular neurothekeoma consists of nests and fascicles of epithelioid cells with vesicular nuclei that contain scant myxoid stroma.
Desmoplastic and atypical variants of cellular neurothekeoma have been described. [18, 19] In a study of 133 cellular neurothekeomas, 74 cases (56%) demonstrated at least one atypical feature. [6] Mitotic activity may be present but is usually low, with a mean of 3 per 10 high power fields (HPF) reported in one study. [6]
Perineural and vascular invasion may rarely be seen in cellular neurothekeoma. Cellular neurothekeoma can be histologically mistaken for melanoma and is, therefore, important to recognize. Also included in the histologic differential diagnosis are Spitz nevi, smooth muscle tumors, fibrohistiocytic tumors, and carcinomas.
A study by Stratton et al of the histologic features of cellular neurothekeoma found that a clue to diagnosis is the presence of nests of epithelioid tumor cells with characteristic cytologic features. [17]
Intermediate subtype
Tumors with features of both cellular and myxoid variants are termed intermediate type. In contrast to the random growth pattern seen in myxoid neurothekeoma, cellular and intermediate variants demonstrate a whorled growth pattern. See the images below.
 Higher power of the cellular neurothekeoma in the previous image demonstrating a whorled growth pattern.
Higher power of the cellular neurothekeoma in the previous image demonstrating a whorled growth pattern.
Immunohistochemistry
The myxoid neurothekeoma subtype demonstrates consistent diffuse S100 protein and glial fibrillary acidic protein (GFAP) immunoreactivity, supporting its schwannian differentiation. The cellular subtype has been shown to demonstrate focal positivity for S100 protein in a minority cases. [20, 21]
NK1/C3, an antibody that stains neuroectodermal tissue, has been shown to stain cellular neurothekeoma. [22] PGP9.5, a broad marker for neuroectodermally derived tumors, also demonstrates positivity in cellular neurothekeoma and PRAME positivity has been reported. [21] However, these markers are not specific for cellular neurothekeoma and stain other lesions which enter into its histopathologic differential. For this reason NK1/C3 and PGP9.5 are not helpful when making a close distinction.
S100 A6 is a member of the S100 protein family that has been demonstrated to have very high sensitivity for cellular neurothekeoma. [20, 23] However, this marker has a low specificity, as it also expressed in melanoma, nevi, neuroblastoma, carcinoma, and fibrohistiocytic tumors. [20] Plaza and coworkers reported 100% sensitivity for S100A6 in their study of 31 cellular neurothekeomas and suggested that immunolabeling of S100A6 in the absence of S100 protein and cytokeratin provides a useful panel in diagnosing cellular neurothekeoma. [20]
Sixty percent of cellular neurothekeomas have shown positivity for smooth muscle actin (SMA); cellular neurothekeomas are usually diffusely positive for neuron-specific enolase (NSE).
Recurrence and Predictive Factors
Although neurothekeoma is considered a benign neoplasm, due to the possibility of recurrence and local invasion, it is recommended that wide local excision with frozen-section–margin control be employed for treatment. [14]
In a follow-up study of 85 neurothekeomas, 13 patients demonstrated lesion regrowth. [3] It should be noted, however, that definitive surgical treatment of the studied lesions was not known. Patients with tumor regrowth were more likely to be younger, female, have facial lesions, and a myxoid pattern on pathology. [3]
In a different follow-up study of 69 cases of cellular neurothekeoma, 10 tumors recurred locally. [6] No tumor recurred more than once, and all recurring cases had been marginally excised or had involved excision margins. The only features that correlated with recurrence were positive excision margins and head and neck location. [6] No metastases were reported.
Follow-up studies of atypical cellular neurothekeomas have not shown that the presence of increased number of mitoses or cellular atypia correlates with any clinically significant endpoints. [6] Large tumor size, atypical histologic features (high mitotic rate, pleomorphism, infiltration of adipose) have been shown to have no clinical significance, but caution is advised as malignant spindle cell tumors may overlap histologically with cellular neurothekeoma.
-
This myxoid neurothekeoma displays a lobulated, nonencapsulated but well-circumscribed proliferation of spindled and epithelioid cells embedded within a myxoid stroma.
-
Higher power view of the myxoid neurothekeoma from the previous image demonstrating the random growth pattern.
-
Cellular neurothekeoma consists of nests and fascicles of epithelioid cells with vesicular nuclei that contain scant myxoid stroma
-
Higher power of the cellular neurothekeoma in the previous image demonstrating a whorled growth pattern.
-
An intermediate-type neurothekeoma demonstrates features of both cellular and myxoid variants.
-
Neurothekeoma Pathology. Cellular neurothekeoma consists of nests and fascicles of epithelioid cells with vesicular nuclei that contain scant myxoid stroma.




