Traditional Uses, Pharmacology and Phytochemistry of the Medicinal Plant Flueggea virosa (Roxb. ex Willd.) Royle
Abstract
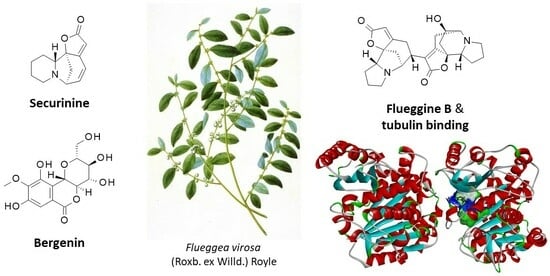
:1. Introduction
2. Pharmacological Effects of F. virosa Extracts
2.1. Antiparasitic Activities
2.2. Antimicrobial Effects
2.3. Antiepilepsy and Antipsychotic Activities
2.4. Antidiabetic Effects
2.5. Antidiabetic Effects
2.6. Anticancer Effects
2.7. Antioxidant Effects
2.8. Other Activities
3. Phytochemical Analyses of F. virosa Extracts
3.1. Polyphenols and Flavonoids
3.2. Terpenoids
3.3. Alkaloids
3.4. Other Compounds
4. Discussion and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, C.; van Welzen, P.C. Flueggea (Euphorbiaceae s. l. or Phyllanthaceae) in Malesia. System Bot. 2010, 35, 541–551. [Google Scholar]
- Wu, Z.L.; Huang, X.J.; Xu, M.T.; Ma, X.; Li, L.; Shi, L.; Wang, W.J.; Jiang, R.W.; Ye, W.C.; Wang, Y. Flueggeacosines A-C, Dimeric Securinine-Type Alkaloid Analogues with Neuronal Differentiation Activity from Flueggea suffruticosa. Org. Lett. 2018, 20, 7703–7707. [Google Scholar] [PubMed]
- Soysa, P.; De Silva, I.S.; Wijayabandara, J. Evaluation of antioxidant and antiproliferative activity of Flueggea leucopyrus Willd (katupila). BMC Complement. Altern. Med. 2014, 14, 274. [Google Scholar]
- Mendis, A.S.; Thabrew, I.; Samarakoon, S.R.; Tennekoon, K.H. Modulation of expression of heat shock proteins and apoptosis by Flueggea leucopyrus (Willd) decoction in three breast cancer phenotypes. BMC Complement. Altern. Med. 2015, 15, 404. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gilbert, M.G.; Fischer, G.; Meyer, C.A. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; Volume 11, p. 178. [Google Scholar]
- Zhang, H.; Zhu, K.K.; Han, Y.S.; Luo, C.; Wainberg, M.A.; Yue, J.M. Flueggether A and Virosinine A, Anti-HIV Alkaloids from Flueggea virosa. Org. Lett. 2015, 17, 6274–6277. [Google Scholar] [CrossRef] [PubMed]
- Tabuti, J.R.S. Flueggea virosa (Roxb. ex Willd.) Voigt. In Plant Resources of Tropical Africa 11(1): Medicinal Plants 1; Schmelzer, G.H., Gurib-Fakim, A., Eds.; Prota Foundation: Wageningen, The Netherlands, 2008; pp. 305–308. Available online: http://database.prota.org/PROTAhtml/Flueggea%20virosa_En.html (accessed on 15 December 2023).
- d’Errico, F.; Backwell, L.; Villa, P.; Degano, I.; Lucejko, J.J.; Bamford, M.K.; Higham, T.F.; Colombini, M.P.; Beaumont, P.B. Early evidence of San material culture represented by organic artifacts from Border Cave, South Africa. Proc. Natl. Acad. Sci. USA 2012, 109, 13214–13219. [Google Scholar] [CrossRef]
- Wang, H.T.; Wang, H.X.; Zhu, Z.X.; Wang, H.F. Complete plastome sequence of Flueggea virosa (Roxburgh ex Willdenow) Voigt (Phyllanthaceae): A medicinal plant. Mitochondrial DNA B Resour. 2020, 5, 2650–2651. [Google Scholar]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar]
- Available online: https://tropical.theferns.info/viewtropical.php?id=Flueggea+virosa (accessed on 15 December 2023).
- Fah, L.; Klotoé, J.R.; Dougnon, V.; Koudokpon, H.; Fanou, V.B.A.; Dandjesso, C.; Loko, F. Étude ethnobotanique des plantes utilisées dans le traitement du diabète chez les femmes enceintes à Cotonou et Abomey-Calavi (Bénin). J. Animal Plant Sci. 2013, 18, 2647–2658. [Google Scholar]
- Sanon, S.; Gansane, A.; Ouattara, L.P.; Traore, A.; Ouedraogo, I.N.; Tiono, A.; Taramelli, D.; Basilico, N.; Sirima, S.B. In vitro antiplasmodial and cytotoxic properties of some medicinal plants from western Burkina Faso. Afr. J. Lab. Med. 2013, 2, 81. [Google Scholar]
- Ouôba, P.; Lykke, A.M.; Boussim, J.; Guinko, S. La flore médicinale de la Forêt Classée de Niangoloko (Burkina Faso). Etudes Flor. Vég. Burkina Faso 2006, 10, 5–16. [Google Scholar]
- Malzy, P. Quelques plantes du Nord Cameroun et leurs utilisations. J. D’agriculture Trop. Bot. Appliquée 1954, 1, 148–179. [Google Scholar]
- Boulesteix, M.; Guinko, S. Plantes Médicinales Utilisées par les Gbayas dans la Région de Bouar (Empire Centrafricain); Quatrième colloque du Conseil Africain de Malgache pour L’enseignement Supérieur (C.A.M.E.S.): Libreville, Gabon, 1979; pp. 23–52. [Google Scholar]
- Terashima, H.; Ichikawa, M. A comparative ethnobotany of the Mbuti and Efe hunter-gatherers in Itury forest, Democratic Republic of Congo. Afr. Study Monogr. 2003, 24, 1–168. [Google Scholar]
- Teklehaymanot, T.; Giday, M. Ethnobotanical study of wild edible plants of Kara and Kwego semi-pastoralist people in Lower Omo River Valley, Debub Omo Zone, SNNPR, Ethiopia. J. Ethnobiol. Ethnomed. 2010, 6, 23. [Google Scholar]
- Wondimu, T.; Asfaw, Z.; Kelbessa, E. Ethnobotanical study of medicinal plants around ‘Dheeraa’ town, Arsi Zone, Ethiopia. J. Ethnopharmacol. 2007, 112, 152–161. [Google Scholar] [PubMed]
- Asase, A.; Akwetey, G.A.; Achel, D.G. Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J Ethnopharmacol 2010, 129, 367–376. [Google Scholar]
- Renu, S.N.; Rawat, A.; Kaur, J.; Kumar, S.; Fatima, N. Taxonomy, phytochemistry, pharmacology and traditional uses of Flueggea virosa (Roxb. ex Willd.) Royle: A Review. Int. J. Life Sci. 2018, 6, 579–585. [Google Scholar]
- Satpute, S.V.; Sinkar, S.R.; Sarode, A.M. Wild edible fruit plants and their use by tribal people and local villagers: A survey-based study, Int. Res. J. Sci. Eng. 2021, A11, 256–262. [Google Scholar]
- Ambe, G.A.; Malaisse, F. Diversité des plantes médicinales et ethnotaxonomie en pays Malinké de Côte d’Ivoire. In Des Sources du Savoir aux Médicaments du Futur: Actes du 4e Congrès Européen D’ethnopharmacologie = From the Sources of Knowledge to the Medicines of the Future: Proceedings of the 4th European Congress on Ethnopharmocology; Fleurentin, J., Pelt, J.M., Mazars, G., Eds.; Lejosne J.C. (trad.), Cabalion Pierre (collab.); Paris (FRA); Metz: IRD; SFE; Congrès Européen d’Ethnopharmacologie, 4; FRA: Metz, France, 2000; pp. 331–338. ISBN 2-7099-1504-9. [Google Scholar]
- Muthaura, C.N.; Rukunga, G.M.; Chhabra, S.C.; Mungai, G.M.; Njagi, E.N. Traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J. Ethnopharmacol. 2007, 114, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Muthaura, C.N.; Rukunga, G.M.; Chhabra, S.C.; Omar, S.A.; Guantai, A.N.; Gathirwa, J.W.; Tolo, F.M.; Mwitari, P.G.; Keter, L.K.; Kirira, P.G.; et al. Antimalarial activity of some plants traditionally used in treatment of malaria in Kwale district of Kenya. J. Ethnopharmacol. 2007, 112, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Muthaura, C.N.; Keriko, J.M.; Mutai, C.; Yenesew, A.; Gathirwa, J.W.; Irungu, B.N.; Nyangacha, R.; Mungai, G.M.; Derese, S. Antiplasmodial potential of traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J. Ethnopharmacol. 2015, 170, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kareru, P.G.; Kenji, G.M.; Gachanja, A.N.; Keriko, J.M.; Mungai, G. Traditional medicines among the Embu and Mbeere peoples of Kenya. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 75–86. [Google Scholar]
- Wanzala, W.; Takken, W.; Mukabana, W.R.; Pala, A.O.; Hassanali, A. Ethnoknowledge of Bukusu community on livestock tick prevention and control in Bungoma district, western Kenya. J. Ethnopharmacol. 2012, 140, 298–324. [Google Scholar] [PubMed]
- Gathirwa, J.W.; Rukunga, G.M.; Mwitari, P.G.; Mwikwabe, N.M.; Kimani, C.W.; Muthaura, C.N.; Kiboi, D.M.; Nyangacha, R.M.; Omar, S.A. Traditional herbal antimalarial therapy in Kilifi district, Kenya. J. Ethnopharmacol. 2011, 134, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.P. Plantes médicinales du Nord de Madagascar. In Ethnobotanique Antakarana et Informations Scientifiques; du Monde, J., Ed.; Jardins du Monde: Brasparts, France, 2012; 296p. [Google Scholar]
- Bah, S.; Diallo, D.; Dembélé, S.; Paulsen, B.S. Ethnopharmacological survey of plants used for the treatment of schistosomiasis in Niono District, Mali. J. Ethnopharmacol. 2006, 105, 387–399. [Google Scholar] [PubMed]
- Danton, O.; Somboro, A.; Fofana, B.; Diallo, D.; Sidibés, L.; Rubat-Coudert, C.; Marchand, F.; Eschalierd, A.; Ducki, S.; Chalard, P. Ethnopharmacological survey of plants used in the traditional treatment of pain conditions in Mali. J. Herbal. Med. 2019, 17–18, 100271. [Google Scholar]
- Van der Steur, L. Plantes médicinales utilisées par les Peul du Sénégal Oriental. Rev. Med. Pharm. Afr. 1994, 8, 189–200. [Google Scholar]
- Inngjerdingen, K.; Nergård, C.S.; Diallo, D.; Mounkoro, P.P.; Paulsen, B.S. An ethnopharmacological survey of plants used for wound healing in Dogonland, Mali, West Africa. J. Ethnopharmacol. 2004, 92, 233–244. [Google Scholar] [PubMed]
- Sanogo, R. Medicinal plants traditionally used in Mali for dysmenorrhea. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 90–96. [Google Scholar] [CrossRef]
- Ribeiro, A.; Romeiras, M.M.; Tavares, J.; Faria, M.T. Ethnobotanical survey in Canhane village, district of Massingir, Mozambique: Medicinal plants and traditional knowledge. J. Ethnobiol. Ethnomed. 2010, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.G.; Echard, N.; Lescot, M. Plantes médicinales Hausa de l’Ader (République du Niger). J. Agric. Trop. Bot. Appl. (JATBA) 1972, 19, 259–399. [Google Scholar] [CrossRef]
- Magaji, M.G.; Yaro, A.H.; Musa, A.M.; Anuka, J.A.; Abdu-Aguye, I.; Hussaini, I.M. Central depressant activity of butanol fraction of Securinega virosa root bark in mice. J. Ethnopharmacol. 2012, 141, 128–133. [Google Scholar] [PubMed]
- Soladoye, M.O.; Ikotun, T.; Chukwuma, E.C.; Ariwaodo, J.O.; Ibhanesebor, G.A.; Agbo-Adediran, O.A.; Owolabi, S.M. Our plants, our heritage: Preliminary survey of some medicinal plant species of Southwestern University Nigeria Campus, Ogun State, Nigeria. Ann. Biol. Res. 2013, 4, 27–34. [Google Scholar]
- Kerharo, J. Senegalese pharmacopoeia: Catalog of medicinal and toxic plants of Wolof and Serer, augmented by the mention of common and vernacular names, of properties and uses, generally recognized in traditional medicine. Ann. Pharm. Fr. 1967, 25, 385–438. [Google Scholar]
- Kerharo, J.; Adam, J.G. Plantes médicinales et toxiques des Peuls et des Toucouleurs du Sénégal. J. Agric. Trop. Bot. Appl. (JATBA) 1964, 11, 384–444+543–599. [Google Scholar]
- Samuelsson, G.; Farah, M.H.; Claeson, P.; Hagos, M.; Thulin, M.; Hedberg, O.; Warfa, A.M.; Hassan, A.O.; Elmi, A.H.; Abdurahman, A.D.; et al. Inventory of plants used in traditional medicine in Somalia. II. Plants of the families Combretaceae to Labiatae. J. Ethnopharmacol. 1992, 37, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Mashile, S.P.; Tshisikhawe, M.P.; Masevhe, N.A. Indigenous fruit plants species of the Mapulana of Ehlanzeni district in Mpumalanga province, South Africa. S. Afr. J. Bot. 2019, 122, 180–183. [Google Scholar] [CrossRef]
- deWet, H.; Ngubane, S.C. Traditional herbal remedies used by women in a rural community in northern Maputaland (South Africa) for the treatment of gynaecology and obstetric complaints. S. Afr. J. Bot. 2014, 94, 129–139. [Google Scholar] [CrossRef]
- Mongalo, N.I.; Makhafola, T.J. Ethnobotanical knowledge of the lay people of Blouberg area (Pedi tribe), Limpopo Province, South Africa. J. Ethnobiol. Ethnomed. 2018, 14, 46. [Google Scholar] [CrossRef]
- Rasethe, M.T.; Semenya, S.S.; Potgieter, M.J.; Maroyi, A. The utilization and management of plant resources in rural areas of the Limpopo Province, South Africa. J. Ethnobiol. Ethnomed. 2013, 9, 27. [Google Scholar] [CrossRef]
- Moshi, M.J.; Uiso, F.C.; Mahunnah, R.L.A.; Mbwanbo, Z.H.; Kapingu, M.C. A survey of plants used by traditional healers in the management of non-insulin dependent diabetes mellitus. East Cent. Afr. J. Pharm. Sci. 2000, 3, 30–39. [Google Scholar]
- Haerdi, F. Afrikanische Heilpflanzen. Die Eingeborenen-Heilpflanzen des Ulanga- Distriktes Tanganjikas (Ostafrika). Acta Trop. 1964, S8, 1–278. [Google Scholar]
- Moshi, M.J.; Otieno, D.F.; Weisheit, A. Ethnomedicine of the Kagera Region, north western Tanzania. Part 3: Plants used in traditional medicine in Kikuku village, Muleba District. J. Ethnobiol. Ethnomed. 2012, 8, 14. [Google Scholar]
- Hedberg, I.; Hedberg, O.; Madati, P.J.; Mshigeni, K.E.; Mshiu, E.N.; Samuelsson, G. Inventory of plants used in traditional medicine in Tanzania. I. Plants of the families Acanthaceae-Cucurbitaceae. J. Ethnopharmacol. 1982, 6, 29–60. [Google Scholar] [PubMed]
- Amri, E.; Kisangau, D.P. Ethnomedicinal study of plants used in villages around Kimboza forest reserve in Morogoro, Tanzania. J. Ethnobiol. Ethnomed. 2012, 8, 1. [Google Scholar] [CrossRef]
- Chhabra, S.C.; Mahunnah, R.L.; Mshiu, E.N. Plants used in traditional medicine in eastern Tanzania. III. Angiosperms (Euphorbiaceae to Menispermaceae). J. Ethnopharmacol. 1990, 28, 255–283. [Google Scholar] [CrossRef] [PubMed]
- Kpodar, M.S.; Karou, S.D.; Katawa, G.; Anani, K.; Gbekley, H.E.; Adjrah, Y.; Tchacondo, T.; Batawila, K.; Simpore, J. An ethnobotanical study of plants used to treat liver diseases in the Maritime region of Togo. J. Ethnopharmacol. 2016, 181, 263–273. [Google Scholar] [CrossRef]
- Kamatenesi-Mugisha, M.; Oryem-Origa, H. Traditional herbal remedies used in the management of sexual impotence and erectile dysfunction in western Uganda. Afr. Health Sci. 2005, 5, 40–49. [Google Scholar] [PubMed]
- Tabuti, J.R.; Lye, K.A.; Dhillion, S.S. Traditional herbal drugs of Bulamogi, Uganda: Plants, use and administration. J. Ethnopharmacol. 2003, 88, 19–44. [Google Scholar] [PubMed]
- Tabuti, J.R. Herbal medicines used in the treatment of malaria in Budiope county, Uganda. J. Ethnopharmacol. 2008, 116, 33–42. [Google Scholar]
- Ssegawa, P.; Kasenene, J.M. Medicinal plant diversity and uses in the Sango bay area, Southern Uganda. J. Ethnopharmacol. 2007, 113, 521–540. [Google Scholar] [CrossRef]
- Okello, J.; Ssegawa, P. Medicinal plants used by communities of Ngai Subcounty, Apac District, northern Uganda. Afr. J. Ecol. 2007, 45, 76–83. [Google Scholar]
- Tugume, P.; Kakudidi, E.K.; Buyinza, M.; Namaalwa, J.; Kamatenesi, M.; Mucunguzi, P.; Kalema, J. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J. Ethnobiol. Ethnomed. 2016, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Oryema, C.; Bukenya Ziraba, R.; Omagor, N.; Opio, A. Medicinal plants of Erute county, Lira district, Uganda with particular reference to their conservation. Afr. J. Ecol. 2010, 48, 285–298. [Google Scholar] [CrossRef]
- Maroyi, A. An ethnobotanical survey of medicinal plants used by the people in Nhema communal area, Zimbabwe. J. Ethnopharmacol. 2011, 136, 347–354. [Google Scholar] [CrossRef]
- Maroyi, A. Traditional use of medicinal plants in south-central Zimbabwe: Review and perspectives. J. Ethnobiol. Ethnomed. 2013, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Ajaib, M.; Pullaiah, T.; Shah, S. Phytochemistry and Pharmacology of Flueggea virosa (Roxb. ex Willd.) Royle. In Bioactives and Pharmacology of Medicinal Plants, 1st ed.; Pullaiah, T., Ed.; Apple Academic Press: New York, NY, USA, 2022; Volume 1, Chapter 14; 12p, ISBN 9781003281658. [Google Scholar]
- Ouachinou, J.M.S.; Dassou, G.H.; Azihou, A.F.; Adomou, A.C.; Yédomonhan, H. Breeders’ knowledge on cattle fodder species preference in rangelands of Benin. J. Ethnobiol. Ethnomed. 2018, 14, 66. [Google Scholar]
- Omara, T.; Kagoya, S.; Openy, A.; Omute, T.; Ssebulime, S.; Kiplagat, K.M.; Bongomin, O. Antivenin plants used for treatment of snakebites in Uganda: Ethnobotanical reports and pharmacological evidences. Trop. Med. Health 2020, 48, 6. [Google Scholar] [CrossRef]
- Diallo, D.; Diakité, C.; Mounkoro, P.P.; Sangaré, D.; Graz, B.; Falquet, J.; Giani, S. [Knowledge of traditional healers on malaria in Kendi (Bandiagara) and Finkolo (Sikasso) in Mali]. Mali. Med. 2007, 22, 1–8. [Google Scholar]
- Kaou, A.M.; Mahiou-Leddet, V.; Hutter, S.; Aïnouddine, S.; Hassani, S.; Yahaya, I.; Azas, N.; Ollivier, E. Antimalarial activity of crude extracts from nine African medicinal plants. J. Ethnopharmacol. 2008, 116, 74–83. [Google Scholar]
- Singh, S.V.; Manhas, A.; Kumar, Y.; Mishra, S.; Shanker, K.; Khan, F.; Srivastava, K.; Pal, A. Antimalarial activity and safety assessment of Flueggea virosa leaves and its major constituent with special emphasis on their mode of action. Biomed. Pharmacother. 2017, 89, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Kraft, C.; Jenett-Siems, K.; Siems, K.; Jakupovic, J.; Mavi, S.; Bienzle, U.; Eich, E. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother. Res. 2003, 17, 123–128. [Google Scholar]
- Tajbakhsh, E.; Kwenti, T.E.; Kheyri, P.; Nezaratizade, S.; Lindsay, D.S.; Khamesipour, F. Antiplasmodial, antimalarial activities and toxicity of African medicinal plants: A systematic review of literature. Malar. J. 2021, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Roy, B.; Venkataswamy, M.; Giri, B.R. Effects of Acacia oxyphylla and Securinega virosa on functional characteristics of Raillietina echinobothrida (Phylum: Platyhelminthes; Class: Cestoidea), a poultry cestode parasite. J. Parasit. Dis. 2013, 37, 125–130. [Google Scholar] [PubMed]
- Dasgupta, S.; Giri, B.R.; Roy, B. Ultrastructural observations on Raillietina echinobothrida exposed to crude extract and active compound of Securinega virosa. Micron 2013, 50, 62–67. [Google Scholar] [CrossRef]
- Freiburghaus, F.; Ogwal, E.N.; Nkunya, M.H.; Kaminsky, R.; Brun, R. In vitro antitrypanosomal activity of African plants used in traditional medicine in Uganda to treat sleeping sickness. Trop. Med. Int. Health 1996, 1, 765–771. [Google Scholar] [CrossRef]
- Nyasse, B.; Nono, J.; Sonke, B.; Denier, C.; Fontaine, C. Trypanocidal activity of bergenin, the major constituent of Flueggea virosa, on Trypanosoma brucei. Pharmazie 2004, 59, 492–494. [Google Scholar]
- El-Hawary, S.S.; Mohammed, R.; AbouZid, S.; Zaki, M.A.; Ali, Z.Y.; Elwekeel, A.; Elshemy, H.A.H. Antitrypanosomal activity of new semi-synthetic bergenin derivatives. Chem. Biol. Drug Des. 2022, 99, 179–186. [Google Scholar]
- Elsheikh, S.H.; Bashir, A.K.; Suliman, S.M.; El Wassila, M. Toxicity of certain Sudanese Plant Extracts to Cercariae and Miracidia of Schistosoma mansoni. Int. J. Crude Drug Res. 1990, 28, 241–245. [Google Scholar] [CrossRef]
- Dickson, R.A.; Houghton, P.J.; Hylands, P.J.; Gibbons, S. Antimicrobial, resistance-modifying effects, antioxidant and free radical scavenging activities of Mezoneuron benthamianum Baill., Securinega virosa Roxb. &Wlld. and Microglossa pyrifolia Lam. Phytother. Res. 2006, 20, 41–45. [Google Scholar]
- Oghenemaro, E.F.; Oise, I.E.; Cynthia, D. The Effects of Securinega virosa Leaves on Methicillin-Resistant Staphylococcus aureus (MRSA). Int. J. Pharm. Res. Allied. Sci. 2021, 10, 29–34. [Google Scholar] [CrossRef]
- Amenu, J.D.; Neglo, D.; Abaye, D.A. Comparative Study of the Antioxidant and Antimicrobial Activities of Compounds Isolated from Solvent Extracts of the Roots of Securinega virosa. J. Biosci. Med. 2019, 7, 27–41. [Google Scholar]
- Anarado, C.E.; Anarado, C.J.O.; Umedum, N.L.; Chukwubueze, F.M.; Anarado, I.L. Phytochemical and Antimicrobial analysis of leaves of Bridelia micrantha, Cassytha filiformis, Euphorbia hirta and Securinega virosa. J. Pharmacogn. Phytochem. 2020, 9, 581–587. [Google Scholar]
- Pedersen, M.E.; Vestergaard, H.T.; Hansen, S.L.; Bah, S.; Diallo, D.; Jäger, A.K. Pharmacological screening of Malian medicinal plants used against epilepsy and convulsions. J. Ethnopharmacol. 2009, 121, 472–475. [Google Scholar] [PubMed]
- Magaji, M.G.; Anuka, J.A.; Abdu-Aguye, I.; Yaro, A.H.; Hussaini, I.M. Preliminary studies on anti-inflammatory and analgesic activities of Securinega virosa (Euphorbiaceae) in experimental animal models. J. Med. Plants Res. 2008, 2, 39–44. [Google Scholar]
- Magaji, M.G.; Yakubu, Y.; Magaji, R.A.; Musa, A.M.; Yaro, A.H.; Hussaini, I.M. Psychopharmacological potentials of methanol leaf extract of Securinega virosa Roxb (Ex Willd) Baill. in mice. Pak. J. Biol. Sci. 2014, 17, 855–859. [Google Scholar] [CrossRef]
- Aiyelero, A.M.; Abdu-Aguye, S.N.; Yaro, A.H.; Magaji, M.G. Behavioural studies on the methanol leaf extract of Securiga virosa (Euphorbiaceae) in mice. J. Pharmacogn. Phythother 2012, 4, 12–15. [Google Scholar]
- Magaji, M.G.; Yaro, A.H.; Musa, A.M.; Anuka, J.A.; Abdu-Aguye, I.; Hussaini, I.M. Sedative activity of residual aqueous fraction of Securinega virosa (Roxb. exWilld) Baill. Root bark extract in mice. Niger. J. Pharm. Sci. 2011, 10, 34–44. [Google Scholar]
- Magaji, M.G.; Mohammed, M.; Magaji, R.A.; Musa, A.M.; Abdu-Aguye, I.; Hussaini, I.M. Evaluation of the antipsychotic potential of aqueous fraction of Securinega virosa root bark extract in mice. Metab. Brain Dis. 2014, 29, 161–165. [Google Scholar] [CrossRef]
- Magaji, M.G.; Musa, A.M.; Abdullahi, M.I.; Ya’u, J.; Hussaini, I.M. Isolation of bergenin from the root bark of Securinega virosa and evaluation of its potential sleep promoting effect. Avicenna J. Phytomed 2015, 5, 587–596. [Google Scholar] [PubMed]
- Chauke, A.M.; Shai, L.J.; Mphahlele, P.M.; Mogale, M.A. Radical scavenging activity of selected medicinal plants from Limpopo province of South Africa. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, A.; Sharma, A. Antianxiety activity guided isolation and characterization of bergenin from Caesalpinia digyna Rottler roots. J. Ethnopharmacol. 2017, 195, 182–187. [Google Scholar] [PubMed]
- Defeudis, G.; Mazzilli, R.; Tenuta, M.; Rossini, G.; Zamponi, V.; Olana, S.; Faggiano, A.; Pozzilli, P.; Isidori, A.M.; Gianfrilli, D. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab. Res. Rev. 2022, 38, e3494. [Google Scholar] [PubMed]
- Moshi, M.J.; Kapingu, M.C.; Uiso, F.C.; Mbwambo, Z.H.; Mahunnah, R.L. Some pharmacological properties of an aqueous extract of Securinega virosa roots. Pharm. Biol. 2000, 38, 214–221. [Google Scholar] [PubMed]
- Tanko, Y.; Okasha, M.A.; Magaji, G.M.; Yerima, M.; Yaro, A.H.; Saleh, M.I.A.; Mohammed, A. Anti-diabetic properties of Securinega virosa (Euphorbiaceae) leaf extract. Afr. J. Biotechnol. 2008, 7, 22–24. [Google Scholar]
- Deshmukh, M.V.; Ghole, V.S.; Kodam, K.M. Protective Effect Of Hydro-Ethanolic Extract Of Bushweed Flueggea virosa On Renal Damage In Streptozotocin-Induced Hyperglycemia In Rat. J. Pharm. Neg. Res. 2022, 13, 6675–6687. [Google Scholar]
- Adinortey, M.B.; Agbeko, R.; Boison, D.; Ekloh, W.; Kuatsienu, L.E.; Biney, E.E.; Affum, O.O.; Kwarteng, J.; Nyarko, A.K. Phytomedicines Used for Diabetes Mellitus in Ghana: A Systematic Search and Review of Preclinical and Clinical Evidence. Evid. Based Complement. Alternat Med. 2019, 2019, 6021209. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, P.; Kashyap, H.; Gupta, S. Natural Phyto-Active Antihyperglycemic Moieties as Preventive Therapeutics for Diabetes Type II. Plant Sci. Today 2019, 6 (Suppl. S1), 1–5. [Google Scholar] [CrossRef]
- Application of Fluevirosines A in Preparation of Blood Sugar Reducing Medicines. Chinese Patent CN103479625B; Qingdao Municipal Hospital, Qingdao, China, 20 May 2015.
- Magaji, M.G.; Anuka, J.A.; Abdu-Aguye, I.; Yaro, A.H.; Hussaini, I.M. Behavioural effects of the methanolic root bark extract of Securinega virosa in rodents. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 147–153. [Google Scholar] [CrossRef]
- Yerima, M.; Magaji, M.G.; Yaro, A.H.; Tanko, Y.; Mohammed, M.M. Analgesic and anti-inflammatory activities of the methanolic leaves extract of Securinega virosa (Euphorbiaceae). Nigerian J. Pharm. Sci. 2009, 8, 47–53. [Google Scholar]
- Ezeonwumelu, J.O.C.; Matuki, E.K.; Ajayi, A.M.; Okoruwa, A.G.; Tanayen, J.K.; Adiukwu, C.P.; Goji, A.D.T.; Dare, S.; Okonkwo, C.O.; Byarugaba, F. Phytochemical Screening, Acute Toxicity and Analgesic Properties of Aqueous Extract of Flueggea virosa’s Root in Rats. Ibnosina J. Med. Biomed. Sci. 2012, 5, 15–21. [Google Scholar]
- Dénou, A.; Haïdara, M.; Diakité, F.; Doumbia, S.; Dembélé, D.L.; Sanogo, R. Phytochemicals and Biological Activities of Flueggea virosa (Phyllanthaceae) Used in the Traditional Treatment of Benign Prostatic Hyperplasia in Mali. J. Diseases Med. Plants 2021, 7, 119–126. [Google Scholar] [CrossRef]
- Misonge, O.J.; Kamindu, G.N.; Wangui, W.S.; Muita, G.M. An ethnobotanical survey of plants used for the treatment and management of cancer in Embu County, Kenya. J. Med. Plants Stud. 2019, 7, 39–46. [Google Scholar]
- Omara, T.; Odero, M.P.; Obakiro, S.B. Medicinal plants used for treating cancer in Kenya: An ethnopharmacological overview. Bull. Nat. Res. Centre 2022, 46, 148. [Google Scholar]
- Salawu, K.M.; Oyerinde, A.A.; Ajaiyeoba, E.O. Phytochemical, antioxidant, cytotoxicity, antiproliferative and antimicrobial studies of Securinega virosa aerial parts extract. J. Pharm. Allied Sci. 2020, 17, 3296–3305. [Google Scholar]
- Ikpefan, E.O.; Ayinde, B.A.; Mudassar, A.; Farooq, A.D. Securinega virosa leaf and root bark extracts: A comparative anti-cancer study against human breast (MCF-7) and lung (NCI-H460) cancer cell lines. Nigerian J. Bot. 2020, 33, 15–23. [Google Scholar]
- Garba, M.M.; Jamilu, Y.; Muhammad, M.A.; Akpojo, A.J.; Ibrahim, A.A.; Marte, H.I. Securinega virosa (Euphorbiaceae) root bark extract inhibits glioblastoma multiforme cell survival in vitro. Afr. J. Pharm. Pharmacol. 2015, 9, 684–693. [Google Scholar]
- Ushie, O.A.; Longbab, B.D.; Kendeson, A.C.; Aasegh, T.J. Antioxidant activities of Flueggea virosa crude extracts. Trends Sci. Technol. J. 2022, 7, 270–274. [Google Scholar]
- Danlami, U.; David, B.M.; Joyce, O.O.; Olutayo, O.; Thomas, S.A. The Antioxidant Potentials and Phytochemical Properties of the Hexane, Ethyl acetate and Ethanolic Extracts of Securinega virosa (Euphorbiaceae) Leaves. J. Applied Pharm. Sci. 2013, 3, 131–133. [Google Scholar]
- Ajaib, M.; Wahla, S.Q.; Shafi, F.; Zahid, M.T.; Siddiqui, M.F.; Abbas, T. Antimicrobial and Antioxidant Screening of Flueggea virosa. Biosci. Res. 2021, 17, 2791–2798. [Google Scholar]
- Zengin, G.; Dall’Acqua, S.; Sinan, K.I.; Uba, A.I.; Sut, S.; Peron, G.; Etienne, O.K.; Kumar, M.; Cespedes-Acuña, C.L.; Alarcon-Enos, J.; et al. Gathering scientific evidence for a new bioactive natural ingredient: The combination between chemical profiles and biological activities of Flueggea virosa extracts. Food Biosci. 2022, 49, 101967. [Google Scholar] [CrossRef]
- Sanogo, R.; Vassallo, A.; Malafronte, N.; Imparato, S.; Russo, A.; Dal Piaz, F. New phenolic glycosides from Securinega virosa and their antioxidant activity. Nat. Prod. Commun. 2009, 4, 1645–1650. [Google Scholar] [CrossRef]
- de Oliveira, G.A.L.; da Silva Oliveira, G.L.; Nicolau, L.A.D.; Mafud, A.C.; Batista, L.F.; Mascarenhas, Y.P.; de Sousa, L.K.M.; David, J.M.; Pinto, L.S.; Alves, C.Q.; et al. Bergenin from Peltophorum dubium: Isolation, Characterization, and Antioxidant Activities in Non-Biological Systems and Erythrocytes. Med. Chem. 2017, 13, 592–603. [Google Scholar]
- Zhang, G.; Wang, H.; Zhang, Q.; Zhao, Z.; Zhu, W.; Zuo, X. Bergenin alleviates H2 O2 -induced oxidative stress and apoptosis in nucleus pulposus cells: Involvement of the PPAR-γ/NF-κB pathway. Environ. Toxicol. 2021, 36, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Thiombiano, H.M.V.; Bangou, M.J.; Nacoulma, A.P.; Ouoba, B.; Sawadogo, M.; Lema, A.; Coulidiati, T.H.; Ouoba, H.Y.; Ouedraogo, G.A. Ethnobotanical survey on medicinal plants used in Burkina Faso in the treatment of breast cancer, phytochemistry and antioxidant activities: Euphorbia poissonii Pax and Flueggea virosa (Willd.) Voigt. (Euphorbiaceae). Afr. J. Biol. Med. Res. 2022, 5, 1–16. [Google Scholar]
- Pu, H.L.; Huang, X.; Zhao, J.H.; Hong, A. Bergenin is the antiarrhythmic principle of Flueggea virosa. Planta Med. 2002, 68, 372–374. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Y.; Cao, G.; Shi, L.; Song, Y.; Shan, W.; Zhang, M.; Li, P.; Zhou, H.; Zhang, B.; et al. Bergenin alleviates myocardial ischemia-reperfusion injury via SIRT1 signaling. Biomed. Pharmacother. 2023, 158, 114100. [Google Scholar] [CrossRef] [PubMed]
- Souleymane, H.D.; Djibo, A.K.; Seyni, S.H.; Zakaria, O.; Botezatu, A.V.; Dinica, R.M.; Ibrahim Maman Laouali, A.; Kouakou, N.D.V. Phytochemical Characterization and In Vitro Evaluation of the Anti-Sickle Cell Activity of Aqueous and Ethanolic Extracts of Two Medicinal Plants from Niger: Flueggea virosa (Roxb. exWilld.) Royle and Kigelia africana (Lam.) Benth. Plants 2023, 12, 3522. [Google Scholar]
- Abere, T.A.; Egharevba, C.O.; Chukwurah, I.O. Pharmacognostic evaluation and antisickling activity of the leaves of Securinega virosa Roxb. ex Willd. (Euphorbiaceae). Afr. J. Biotechnol. 2014, 13, 4040–4045. [Google Scholar]
- Kouangbé, M.A.; Bahi, C.; Tia, H.; Boga Gogo, L.; Edoh, V.; Djama, A.J.; N’Guessan, J.D. Antifungal Activity of Roots Barks Extract of Securinega virosa (Roxb. ex Willd.) Baill and Anogeissus leiocarpa (DC.) Guill. & Perr, Two Plants Used in the Traditional Treatment of Candidiasis in Northern Côte d’Ivoire. Int. J. Biochem. Res. Rev. 2015, 8, 1–11. [Google Scholar]
- Hill, L.; Holdsworth, D.; Small, R. Pharmacological investigations of virosecurinine. PNG Med. J. 1976, 18, 157–161. [Google Scholar]
- Magaji, M.G.; Yaro, A.H.; Mohammed, A.; Zezi, A.U.; Tanko, Y.; Bala, T.Y. Preliminary Antidiarrhoeal Activity of Methanolic Extracts of Securinega virosa (Euphorbiaceae). Afr. J. Biotechnol. 2007, 6, 2752–2757. [Google Scholar] [CrossRef]
- Njume, C.; Goduka, N.I. Treatment of diarrhoea in rural African communities: An overview of measures to maximise the medicinal potentials of indigenous plants. Int. J. Environ. Res. Public. Health 2012, 9, 3911–3933. [Google Scholar]
- Chinsembu, K.C.; Syakalima, M.; Semenya, S.S. Ethnomedicinal plants used by traditional healers in the management of HIV/AIDS opportunistic diseases in Lusaka, Zambia. S.Afr. J. Bot. 2019, 122, 369–384. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.R.; Han, Y.S.; Wainberg, M.A.; Yue, J.M. New Securinega alkaloids with anti-HIV activity from Flueggea virosa. RSC Adv. 2015, 5, 107045–107053. [Google Scholar] [CrossRef]
- Zhang, H.; Han, Y.-S.; Wainberg, M.A.; Yue, J.-M. Anti-HIV Securinega alkaloid oligomers from Flueggea virosa. Tetrahedron 2015, 71, 3671–3679. [Google Scholar] [CrossRef]
- Olaleye, T.; Muse, W.A.; Imeh-Nathaniel, A.; Nathaniel, T.I. Biological effects of Petiveria alliacea and Flueggae virosa on the life cycle of a disease vector (Musca domestica). Int. J. Pure Appl. Zool. 2017, 5, 45–51. [Google Scholar]
- Bajracharya, G.B. Diversity, pharmacology and synthesis of bergenin and its derivatives: Potential materials for therapeutic usages. Fitoterapia 2015, 101, 133–152. [Google Scholar]
- Salimo, Z.M.; Yakubu, M.N.; da Silva, E.L.; de Almeida, A.C.G.; Chaves, Y.O.; Costa, E.V.; da Silva, F.M.A.; Tavares, J.F.; Monteiro, W.M.; de Melo, G.C.; et al. Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule. Biomolecules 2023, 13, 403. [Google Scholar] [CrossRef]
- Dai, W.; Yang, J.; Liu, X.; Mei, Q.; Peng, W.; Hu, X. Anti-colorectal cancer of Ardisia gigantifolia Stapf. and targets prediction via network pharmacology and molecular docking study. BMC Complement. Med. Ther. 2023, 23, 4. [Google Scholar] [CrossRef]
- Lei, D.; Sun, Y.; Liu, J.; Chi, J. Bergenin inhibits palmitic acid-induced pancreatic β-cell inflammatory death via regulating NLRP3 inflammasome activation. Ann. Transl. Med. 2022, 10, 1058. [Google Scholar] [PubMed]
- Venkateswara Rao, B.; Pavan Kumar, P.; Ramalingam, V.; Karthik, G.; Andugulapati, S.B.; Suresh Babu, K. Piperazine tethered bergenin heterocyclic hybrids: Design, synthesis, anticancer activity, and molecular docking studies. RSC Med. Chem. 2022, 13, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, R.S.; Wijewardhane, P.; Herath, C.; Perera, S. Bergenin: A computationally proven promising scaffold for novel galectin-3 inhibitors. J. Mol. Model. 2018, 24, 302. [Google Scholar] [CrossRef] [PubMed]
- Barai, P.; Raval, N.; Acharya, S.; Borisa, A.; Bhatt, H.; Acharya, N. Neuroprotective effects of bergenin in Alzheimer’s disease: Investigation through molecular docking, in vitro and in vivo studies. Behav. Brain Res. 2019, 356, 18–40. [Google Scholar] [CrossRef]
- Madaan, R.; Singla, R.K.; Kumar, S.; Dubey, A.K.; Kumar, D.; Sharma, P.; Bala, R.; Singla, S.; Shen, B. Bergenin—A Biologically Active Scaffold: Nanotechnological Perspectives. Curr. Top. Med. Chem. 2022, 22, 132–149. [Google Scholar]
- Sharma, P.; Kumar, D.; Shri, R.; Kumar, S. Mechanistic Insights and Docking Studies of Phytomolecules as Potential Candidates in the Management of Cancer. Curr. Pharm. Des. 2022, 28, 2704–2724. [Google Scholar]
- Li, G.; Fang, Y.; Ma, Y.; Dawa, Y.; Wang, Q.; Gan, J.; Dang, J. Screening and Isolation of Potential Anti-Inflammatory Compounds from Saxifraga atrata via Affinity Ultrafiltration-HPLC and Multi-Target Molecular Docking Analyses. Nutrients 2022, 14, 2405. [Google Scholar] [CrossRef]
- Wang, G.-C.; Liang, J.-P.; Wang, Y.; Li, Q.; Ye, W.-C. Chemical constituents from Flueggea virosa. Chin. J. Nat. Med. 2008, 6, 251–253. [Google Scholar]
- Wang, X.F.; Liu, F.F.; Zhu, Z.; Fang, Q.Q.; Qu, S.J.; Zhu, W.; Yang, L.; Zuo, J.P.; Tan, C.H. Flueggenoids A–E, new dinorditerpenoids from Flueggea virosa. Fitoterapia 2019, 133, 96–101. [Google Scholar]
- Haq, K.U.; Rusdipoetra, R.A.; Siswanto, I.; Suwito, H. Elucidation of reactive oxygen species scavenging pathways of norbergenin utilizing DFT approaches. R. Soc. Open Sci. 2022, 9, 221349. [Google Scholar] [CrossRef]
- Li, W.; Cai, Z.; Schindler, F.; Bahiraii, S.; Brenner, M.; Heiss, E.H.; Weckwerth, W. Norbergenin prevents LPS-induced inflammatory responses in macrophages through inhibiting NFκB, MAPK and STAT3 activation and blocking metabolic reprogramming. Front. Immunol. 2023, 14, 1117638. [Google Scholar]
- Khan, M.S.; Khan, W.; Ahmad, W.; Singh, M.; Ahmad, S. Bergenin determination in different extracts by high-performance thin-layer chromatographic densitometry. J. Pharm. Bioallied Sci. 2015, 7, 272–274. [Google Scholar]
- Siddiqui, N.A.; Alam, P.; Al-Rehaily, A.J.; Al-Oqail, M.M.; Parvez, M.K. Simultaneous quantification of biomarkers bergenin and menisdaurin in the methanol extract of aerial parts of Flueggea virosa by validated HPTLC densitometric method. J. Chrom Sci. 2015, 53, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Alam, P.; Siddiqui, N.A.; Alajmi, M.F.; Rehman, M.T.; Kalam, M.A.; Al-Rehaily, A.J. Development and validation of UPLC-PDA method for concurrent analysis of bergenin and menisdaurin in aerial parts of Flueggea virosa (Roxb. ex Willd.). Saudi Pharm. J. 2018, 26, 970–976. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Mohammed, R.; Abouzid, S.; Ali, Z.Y.; Elwekeel, A. Anti-arthritic activity of 11-O-(4’-O-methyl galloyl)-bergenin and Crassula capitella extract in rats. J. Pharm. Pharmacol. 2016, 68, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Amin, H.; Ullah, A.; Saba, S.; Rafique, J.; Khan, K.; Ahmad, N.; Badshah, S.L. Antioxidant and Antiplasmodial Activities of Bergenin and 11-O-Galloylbergenin Isolated from Mallotus philippensis. Oxid. Med. Cell Longev. 2016, 2016, 1051925. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, L.; Zhou, L.; Gan, Y.; Han, S.; Zhou, Y.; Qing, X.; Li, W. Bergenin Inhibits Tumor Growth and Overcomes Radioresistance by Targeting Aerobic Glycolysis. Am. J. Chin. Med. 2023, 51, 1905–1925. [Google Scholar]
- Li, X.; Liang, Q.; Zhou, L.; Deng, G.; Xiao, Y.; Gan, Y.; Han, S.; Liao, J.; Wang, R.; Qing, X.; et al. Survivin degradation by bergenin overcomes pemetrexed resistance. Cell. Oncol. 2023, 46, 1837–1853. [Google Scholar] [CrossRef]
- Gan, Y.; Li, X.; Han, S.; Zhou, L.; Li, W. Targeting Mcl-1 Degradation by Bergenin Inhibits Tumorigenesis of Colorectal Cancer Cells. Pharmaceuticals 2023, 16, 241. [Google Scholar] [PubMed]
- Shen, M.; Li, H.; Yuan, M.; Jiang, L.; Zheng, X.; Zhang, S.; Yuan, M. Preparation of bergenin - Poly (lactic acid) polymers and in vitro controlled release studies. Int. J. Biol. Macromol. 2018, 116, 354–363. [Google Scholar]
- Ren, Y.; Shen, M.; Ding, Y.; Yuan, M.; Jiang, L.; Li, H.; Yuan, M. Study on preparation and controlled release in vitro of bergenin-amino polylactic acid polymer. Int. J. Biol. Macromol. 2020, 153, 650–660. [Google Scholar]
- de Sá Hyacienth, B.M.; Tavares Picanço, K.R.; Sánchez-Ortiz, B.L.; Barros Silva, L.; Matias Pereira, A.C.; Machado Góes, L.D.; Sousa Borges, R.; Cardoso Ataíde, R.; Dos Santos, C.B.R.; de Oliveira Carvalho, H.; et al. Hydroethanolic extract from Endopleura uchi (Huber) Cuatrecasas and its marker bergenin: Toxicological and pharmacokinetic studies in silico and in vivo on zebrafish. Toxicol. Rep. 2020, 7, 217–232. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Mothana, R.A.; Al-Rehaily, A.J.; Alam, P.; Yousaf, M.; Ahmed, S.; Alatar, A. High-performance thin-layer chromatography based concurrent estimation of biomarkers ent-phyllanthidine and rutin in the dried aerial parts of Flueggea virosa. Saudi Pharm. J. 2017, 25, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Lin, Y.J.; Cheng, J.C.; Huang, H.C.; Yeh, Y.J.; Wu, T.S.; Hwang, S.Y.; Wu, Y.C. Chemical Constituents from Flueggea virosa and the Structural Revision of Dehydrochebulic Acid Trimethyl Ester. Molecules 2016, 21, 1239. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fisher, D.; Pronyuk, K.; Dang, Y.; Zhao, L. Agent in Urgent Need of Clinical Practice: Corilagin. Mini Rev. Med. Chem. 2023, 23, 1642–1652. [Google Scholar] [PubMed]
- Li, X.; Deng, Y.; Zheng, Z.; Huang, W.; Chen, L.; Tong, Q.; Ming, Y. Corilagin, a promising medicinal herbal agent. Biomed. Pharmacother. 2018, 99, 43–50. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Ganguly, R.; Rana, H.K.; Pandey, P.K.; Sethi, G.; Bishayee, A.; Pandey, A.K. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules 2019, 24, 3399. [Google Scholar]
- Cheng, J.C.; Chen, Y.J.; Chuang, C.W.; Chao, Y.H.; Huang, H.C.; Lin, C.C.; Chao, C.H. Polyoxygenated Terpenoids and Polyketides from the Roots of Flueggea virosa and Their Inhibitory Effect against SARS-CoV-2-Induced Inflammation. Molecules 2022, 27, 8548. [Google Scholar] [CrossRef]
- Chao, C.H.; Cheng, J.C.; Shen, D.Y.; Huang, H.C.; Wu, Y.C.; Wu, T.S. Terpenoids from Flueggea virosa and their anti-hepatitis C virus activity. Phytochemistry 2016, 128, 60–70. [Google Scholar] [CrossRef]
- de Souza, M.M.; Chagas, L.G.R.D.; Gonçalves, A.E.; Tomczak, M.; Reichert, S.; Schuquel, I.T.A.; Cechinel-Filho, V.; Meyre-Silva, C. Phytochemical Analysis and Antinociceptive Properties of Hydroalcoholic Extracts of Aleurites moluccanus Bark. Planta Med. 2021, 87, 896–906. [Google Scholar] [CrossRef]
- Qin, H.; Yu, D. 10.11 Podocarpane-type diterpenoids. In Volume 5 Diterpenoids, Triterpenoids, Sesterterpenoids, Tetraterpenoids, and Carotenoids; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; pp. 196–201. [Google Scholar] [CrossRef]
- Chao, C.H.; Cheng, J.C.; Shen, D.Y.; Wu, T.S. Anti-hepatitis C virus dinorditerpenes from the roots of Flueggea virosa. J. Nat. Prod. 2014, 77, 22–28. [Google Scholar] [CrossRef]
- Chao, C.-H.; Cheng, J.-C.; Hwang, T.-L.; Shen, D.-Y.; Wu, T.-S. Trinorditerpenes from the roots of Flueggea virosa. Bioorg Med. Chemi Lett. 2014, 24, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Harms, K.; Koert, U. Total Syntheses of 7,20-Oxa-Bridged Dinorditerpenes: Antihepatitis C Virus Active (+)-Elevenol from Flueggea virosa and (+)-Przewalskin. Org. Lett. 2016, 18, 5692–5695, Erratum in Org. Lett. 2018, 20, 4158. [Google Scholar] [CrossRef] [PubMed]
- Monkodkaew, S.; Loetchutinat, C.; Nuntasaen, N.; Pompimon, W. Identification and Antiproliferative. Activity Evaluation of a Series of Triterpenoids Isolated from Flueggea virosa (Roxb. ex Willd.) Am. J. Appl. Sci. 2009, 6, 1800–1806. [Google Scholar]
- Aswathy, M.; Vijayan, A.; Daimary, U.D.; Girisa, S.; Radhakrishnan, K.V.; Kunnumakkara, A.B. Betulinic acid: A natural promising anticancer drug, current situation, and future perspectives. J. Biochem. Mol. Toxicol. 2022, 36, e23206. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Sun, Y.; Yuan, B.; Wang, Y. Betulinic acid in the treatment of breast cancer: Application and mechanism progress. Fitoterapia 2023, 169, 105617. [Google Scholar] [CrossRef] [PubMed]
- Okunola, O.J.; Ali, T.; Mann, A.; Bello, O.M. Pharmacological and chemotaxonomic aspect of terpenes in Securinega virosa. FUDMA J. Sci. 2018, 2, 231–237. [Google Scholar]
- Kornel, A.; Nadile, M.; Retsidou, M.I.; Sakellakis, M.; Gioti, K.; Beloukas, A.; Sze, N.S.K.; Klentrou, P.; Tsiani, E. Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2023, 24, 7414. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, F.; Tang, Z.; Yang, X.; Liu, Y.; Wang, F.; Chen, B. Anti-inflammatory and antioxidant activity of ursolic acid: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1256946. [Google Scholar] [CrossRef]
- Muraveva, V.I.; Bankovskii, A.I. Chemical study of alkaloids of Securinega suffruticosa. Dokl. Akda Nauk. SSSR 1956, 110, 998–1000. [Google Scholar]
- Nakano, T.; Yang, T.H.; Terao, S. Studies on the alkaloides of Securinega virosa Pax et Hoff. I. Telmmron. Tetrahedron 1963, 19, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Łuczkiewicz, M. Securinega suffruticosa. Fitoterapia 2008, 79, 419–427. [Google Scholar] [PubMed]
- Chirkin, E.; Atkatlian, W.; Porée, F.H. The Securinega alkaloids. Alkaloids Chem. Biol. 2015, 74, 1–120. [Google Scholar] [PubMed]
- Park, K.J.; Kim, C.S.; Khan, Z.; Oh, J.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Securinega Alkaloids from the Twigs of Securinega suffruticosa and Their Biological Activities. J. Nat. Prod. 2019, 82, 1345–1353. [Google Scholar] [PubMed]
- Hou, W.; Huang, H.; Wu, X.Q.; Lan, J.X. Bioactivities and mechanism of action of securinega alkaloids derivatives reported prior to 2022. Biomed. Pharmacother. 2023, 158, 114190. [Google Scholar]
- Liu, C.J.; Fan, X.D.; Jiang, J.G.; Chen, Q.X.; Zhu, W. Potential anticancer activities of securinine and its molecular targets. Phytomedicine 2022, 106, 154417. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Yang, B.; Hu, J.; Cheng, Y. L-securinine inhibits cell growth and metastasis of human androgen-independent prostate cancer DU145 cells via regulating mitochondrial and AGTR1/MEK/ERK/STAT3/PAX2 apoptotic pathways. Biosci. Rep. 2019, 39, BSR20190469. [Google Scholar] [CrossRef]
- Ashraf, S.M.; Mahanty, S.; Rathinasamy, K. Securinine induces mitotic block in cancer cells by binding to tubulin and inhibiting microtubule assembly: A possible mechanistic basis for its anticancer activity. Life Sci. 2021, 287, 120105. [Google Scholar] [CrossRef] [PubMed]
- Klochkov, S.; Neganova, M. Unique indolizidine alkaloid securinine is a promising scaffold for the development of neuroprotective and antitumor drugs. RSC Adv. 2021, 11, 19185–19195. [Google Scholar] [CrossRef]
- Park, S.; Kang, G.; Kim, C.; Kim, D.; Han, S. Collective total synthesis of C4-oxygenated securinine-type alkaloids via stereocontrolled diversifications on the piperidine core. Nat. Commun. 2022, 13, 5149. [Google Scholar]
- Kang, G.; Park, S.; Han, S. Synthesis of High-Order and High-Oxidation State Securinega Alkaloids. Acc. Chem. Res. 2023, 56, 140–156. [Google Scholar] [CrossRef]
- Iketubosin, G.O.; Mathieson, D.W. The isolation of hordenine and norsecurinine from Securinega virosa. J. Pharm. Pharmacol. 1963, 15, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, W.; Yue, J.-M. From monomer to tetramer and beyond: The intriguing chemistry of Securinega alkaloids from Flueggea virosa. Tetrahedron 2013, 69, 3942–3946. [Google Scholar] [CrossRef]
- Zhao, B.-X.; Wang, Y.; Li, C.; Wang, G.-C.; Huang, X.-J.; Fan, C.-L.; Li, Q.-M.; Zhu, H.-J.; Chen, W.-M.; Ye, W.-C. Flueggedine, a novel axisymmetric indolizidine alkaloid dimer from Flueggea virosa. Tetrahedron Lett. 2013, 54, 4708–4711. [Google Scholar]
- Zhang, H.; Zhang, C.R.; Zhu, K.K.; Gao, A.H.; Luo, C.; Li, J.; Yue, J.M. Fluevirosines A-C: A biogenesis inspired example in the discovery of new bioactive scaffolds from Flueggea virosa. Org. Lett. 2013, 15, 120–123. [Google Scholar] [CrossRef]
- Li, X.-H.; Cao, M.-M.; Zhang, Y.; Li, S.-L.; Di, Y.-T.; Hao, X.-J. Fluevirines A-D, four new securinega-type alkaloids from Flueggea virosa. Tetrahedron Lett. 2014, 55, 6101–6104. [Google Scholar]
- Yang, X.; Liu, J.; Huo, Z.; Yuwen, H.; Li, Y.; Zhang, Y. Fluevirines E and F, two new alkaloids from Flueggea virosa. Nat. Prod. Res. 2020, 34, 2001–2006. [Google Scholar]
- Xie, Q.J.; Zhang, W.Y.; Wu, Z.L.; Xu, M.T.; He, Q.F.; Huang, X.J.; Che, C.T.; Wang, Y.; Ye, W.C. Alkaloid constituents from the fruits of Flueggea virosa. Chin. J. Nat. Med. 2020, 18, 385–392. [Google Scholar]
- Zhao, B.X.; Wang, Y.; Zhang, D.M.; Jiang, R.W.; Wang, G.C.; Shi, J.M.; Huang, X.J.; Chen, W.M.; Che, C.T.; Ye, W.C. Flueggines A and B, two new dimeric indolizidine alkaloids from Flueggea virosa. Org. Lett. 2011, 13, 3888–3891. [Google Scholar] [CrossRef]
- Wei, H.; Qiao, C.; Liu, G.; Yang, Z.; Li, C.C. Stereoselective total syntheses of (-)-flueggine A and (+)-virosaine B. Angew. Chem. Int. Ed. Engl. 2013, 52, 620–624. [Google Scholar] [CrossRef]
- Gan, L.S.; Fan, C.Q.; Yang, S.P.; Wu, Y.; Lin, L.P.; Ding, J.; Yue, J.M. Flueggenines A and B, two novel C,C-linked dimeric indolizidine alkaloids from Flueggea virosa. Org. Lett. 2006, 8, 2285–2288. [Google Scholar]
- Jeon, S.; Lee, J.; Park, S.; Han, S. Total synthesis of dimeric Securinega alkaloids (-)-flueggenines D and I. Chem. Sci. 2020, 11, 10934–10938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, Y.-S.; Wainberg, M.A.; Yue, J.-M. Flueggethers B-D, Securinega alkaloids with rare oligomerizing pattern from Flueggea virosa. Tetrahedron Lett. 2016, 57, 1798–1800. [Google Scholar]
- Luo, X.K.; Cai, J.; Yin, Z.Y.; Luo, P.; Li, C.J.; Ma, H.; Seeram, N.P.; Gu, Q.; Xu, J. Fluvirosaones A and B, Two Indolizidine Alkaloids with a Pentacyclic Skeleton from Flueggea virosa. Org. Lett. 2018, 20, 991–994. [Google Scholar]
- Gan, L.S.; Yue, J.M. Alkaloids from the Root of Flueggea virosa. Nat. Prod. Commun. 2006, 1, 819–823. [Google Scholar] [CrossRef]
- Miyatake-Ondozabal, H.; Bannwart, L.M.; Gademann, K. Enantioselective total synthesis of virosaine A and bubbialidine. Chem. Commun. (Camb) 2013, 49, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Luger, P.; Weber, M.; Dung, N.X.; Ky, P.T.; Long, P.K. Securinine, an Alkaloid from Fluggea virosa. Acta Cryst. 1995, C51, 127–129. [Google Scholar]
- Alibés, R.; Bayón, P.; de March, P.; Figueredo, M.; Font, J.; García-García, E.; González-Gálvez, D. An effective enantioselective approach to the securinega alkaloids: Total synthesis of (-)-norsecurinine. Org. Lett. 2005, 7, 5107–5109. [Google Scholar] [CrossRef]
- González-Gálvez, D.; García-García, E.; Alibés, R.; Bayón, P.; de March, P.; Figueredo, M.; Font, J. Enantioselective approach to Securinega alkaloids. Total synthesis of securinine and (-)-norsecurinine. J. Org. Chem. 2009, 74, 6199–6211. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, K.-K.; Gao, X.-H.; Yue, J.-M. Natural occurrence of all eight stereoisomers of a neosecurinane structure from Flueggea virosa. Tetrahedron 2017, 73, 4692–4697. [Google Scholar] [CrossRef]
- Wehlauch, R.; Grendelmeier, S.M.; Miyatake-Ondozabal, H.; Sandtorv, A.H.; Scherer, M.; Gademann, K. Investigating Biogenetic Hypotheses of the Securinega Alkaloids: Enantioselective Total Syntheses of Secu’amamine E/ent-Virosine A and Bubbialine. Org. Lett. 2017, 19, 548–551. [Google Scholar] [CrossRef]
- Ma, N.; Yao, Y.; Zhao, B.X.; Wang, Y.; Ye, W.C.; Jiang, S. Total synthesis of securinega alkaloids (-)-norsecurinine, (-)-niruroidine and (-)-flueggine A. Chem. Commun. 2014, 50, 9284–9287. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-Y.; Wang, A.-T.; Zhao, B.-X.; Lei, X.-P.; Zhang, D.-M.; Jiang, R.-W.; Wang, Y.; Ye, W.-C. Norsecurinamines A and B, two norsecurinine-derived alkaloid dimers from the fruits of Flueggea virosa. Tetrahedron Lett. 2016, 57, 3810–3813. [Google Scholar] [CrossRef]
- Dehmlow, E.V.; Guntenhöner, M.; Ree, T.V. A novel alkaloid from Flueggea virosa: 14,15-epoxynorsecurinine. Phytochemistry 1999, 52, 1715–1716. [Google Scholar] [CrossRef]
- Tatematsu, H.; Mori, M.; Yang, T.H.; Chang, J.J.; Lee, T.T.; Lee, K.H. Cytotoxic principles of Securinega virosa: Virosecurinine and viroallosecurinine and related derivatives. J. Pharm. Sci. 1991, 80, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-C.; Wang, Y.; Li, Q.; Liang, J.-P.; Zhang, X.-Q.; Yao, X.-S.; Ye, W.-C. Two new alkaloids from Flueggea virosa. Helvet Chim. Acta 2008, 91, 1124–1129. [Google Scholar] [CrossRef]
- Zhao, B.-X.; Wang, Y.; Zhang, D.-M.; Huang, X.-J.; Bai, L.-L.; Yan, Y.; Chen, J.-M.; Lu, T.-B.; Wang, Y.-T.; Zhang, Q.-W.; et al. Virosaines A and B, two new birdcage-shaped Securinega alkaloids with an unprecedented skeleton from Flueggea virosa. Org. Lett. 2012, 14, 3096–3099. [Google Scholar]
- Sertel, S.; Fu, Y.; Zu, Y.; Rebacz, B.; Konkimalla, B.; Plinkert, P.K.; Krämer, A.; Gertsch, J.; Efferth, T. Molecular docking and pharmacogenomics of vinca alkaloids and their monomeric precursors, vindoline and catharanthine. Biochem. Pharmacol. 2011, 81, 723–735. [Google Scholar] [CrossRef]
- Strömbom, J.; Jokela, R.; Saano, V.; Rolfsen, W. Binding of strychnocarpine and related beta-carbolines to brain receptors in vitro. Eur. J. Pharmacol. 1992, 214, 165–168. [Google Scholar] [CrossRef]
- Schmid, C.L.; Bohn, L.M. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ß-arrestin2/Src/Akt signaling complex in vivo. J. Neurosci. 2010, 30, 13513–13524. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants 2023, 12, 296. [Google Scholar] [PubMed]
- Vergoten, G.; Bailly, C. The Plagiochilins from Plagiochila Liverworts: Binding to α-Tubulin and Drug Design Perspectives. AppliedChem 2023, 3, 217–228. [Google Scholar]
- Al-Rehaily, A.J.; Yousaf, M.; Ahmed, M.S.; Samoylenko, V.; Li, X.C.; Muhammad, I.; El Tahir, K.E.H. Chemical and biological study of Flueggea virosa native to Saudi Arabia. Chem. Nat. Compounds 2015, 51, 187–188. [Google Scholar] [CrossRef]
- Muhammad, A.; Sirat, H.M. COX-2 inhibitors from stem bark of Bauhinia rufescens Lam. (Fabaceae). EXCLI J. 2013, 12, 824–830. [Google Scholar] [PubMed]
- Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Ponczek, M.B.; Pecio, Ł.; Nowak, P.; Kolodziejczyk-Czepas, J. Pulmonaria obscura and Pulmonaria officinalis Extracts as Mitigators of Peroxynitrite-Induced Oxidative Stress and Cyclooxygenase-2 Inhibitors-In Vitro and In Silico Studies. Molecules 2021, 26, 631. [Google Scholar] [CrossRef]
- Peng, Y.L.; Zeng, N.; Yao, Q.Y.; Peng, C.Y.; Sheng, W.B.; Li, B.; Wang, W. A Review of the Medicinal Uses, Phytochemistry and Pharmacology of Genus Flueggea. Current Chinese Sci. 2023, 3, 213–241. [Google Scholar] [CrossRef]
- Fouotsa, H.; Le Pogam, P.; Mkounga, P.; Lannang, A.M.; Bernadat, G.; Vanheuverzwijn, J.; Zhou, Z.; Leblanc, K.; Rharrabti, S.; Nkengfack, A.E.; et al. Voatriafricanines A and B, Trimeric Vobasine-Aspidosperma-Aspidosperma Alkaloids from Voacanga africana. J. Nat. Prod. 2021, 84, 2755–2761. [Google Scholar]
- Ma, X.; Chen, H.; Zhu, S.; Tu, P.; Jiang, Y. Trimeric and Dimeric Carbazole Alkaloids from Murraya microphylla. Molecules 2021, 26, 5689. [Google Scholar]
- Wang, Y.; Ma, S.G.; Li, L.; Yu, S.S. Indole alkaloids from the bark of Acacia confusa and their potential antinociceptive and anti-inflammatory activities. J. Asian Nat. Prod. Res. 2022, 24, 1109–1127. [Google Scholar] [CrossRef]
- Kren, V.; Fiserová, A.; Weignerová, L.; Stibor, I.; Halada, P.; Prikrylová, V.; Sedmera, P.; Pospísil, M. Clustered ergot alkaloids modulate cell-mediated cytotoxicity. Bioorg Med. Chem. 2002, 10, 415–424. [Google Scholar] [CrossRef]
- Kren, V.; Eich, E.; Pertz, H.H. Pergolide, terguride and N,N’-spacer-linked oligomers of both interact with 5-HT2A receptors of rat tail artery. Physiol. Res. 2004, 53, 35–43. [Google Scholar] [CrossRef]
- Tang, G.; Liu, X.; Ma, N.; Huang, X.; Wu, Z.L.; Zhang, W.; Wang, Y.; Zhao, B.X.; Wang, Z.Y.; Ip, F.C.; et al. Design and Synthesis of Dimeric Securinine Analogues with Neuritogenic Activities. ACS Chem. Neurosci. 2016, 7, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Wang, Z.Y.; Lin, J.; Chen, W.M. Induction of differentiation of the acute myeloid leukemia cell line (HL-60) by a securinine dimer. Cell Death Discov. 2020, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhuang, X.; Huang, X.; Peng, Y.; Ma, X.; Huang, Z.X.; Liu, F.; Xu, J.; Wang, Y.; Chen, W.M.; et al. A Bivalent Securinine Compound SN3-L6 Induces Neuronal Differentiation via Translational Upregulation of Neurogenic Transcription Factors. Front. Pharmacol. 2018, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.A.; Houghton, P.J.; Govindarajan, R. In-vitro and in-vivo wound healing properties of two plants from Ghana. Planta Med. 2007, 73, 465. [Google Scholar]








|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Country (Region or Province) | Vernacular Names (Language) | References |
|---|---|---|
| English names | White berry bush, snowberry tree, Chinese waterberry, simpleleaf bushweed, common bushweed. | (a) |
| Benin (Cotonou, Abomey-Calavi) | Gaagah, tchian tchian, sian sian (Bariba); yéri kalawunfa (Berba); adjaya, gbyihountin, hétré, hounsividjayé, tchaké tchaké (Fon, Goun); koukrinou (Gourmantché); tchamanoudira (Kotokoli); hesré (Watchi); igi agbado, irandjé, wadjidji (Watchi); hesre (Fon) | [12] |
| Burkina Faso (Ouagadougou) (Niangoloko forest) | Sûgdendaaqa (Mossi), fiŋyaŋma (Goin), sï’ngnamâ (Goin) | [13,14] |
| Burundi (Zaïre-Nil peaks region and Western Burundi) | Umubwirrwa, umubwiga, umujisharugi (Kirundi) | (a) |
| Cameroon (northern region) | Tiami (Fulfuldé) | [15] |
| Central African Republic (Gbaya dialect, Bossangoa) | Marro | [16] |
| Comores | M’haniba (Grande Comores) | (a) |
| Democratic Republic of Congo (Itury forest) | Njima (Mbuti pygmies) | [17] |
| Ethiopia (Omo Zone), (Arsi Zone) | Tania, qacaaculee | [18,19] |
| Fiji | Pou | [1] |
| France | Balan des savanes | (a) |
| Ghana (Dangme West district) | Asre (Adangbe) | [20] |
| India | Perimklavu, vellapoolam (Malayalam); pithondi | [21,22] |
| Ivory Cost | Môgôcolo-calaman (Dioula); mokokoana, mokrodoma (Malinké) | [23] |
| Kenya (Bungoma district), (Kilifi district) | Lubwili (Bukusu); mukwamba (Giriama); kiptarpotich, chepochepkai (Pokot); mkwamba (Swahili); mukuluu (Kamba); lkirebuki (Dorobo) (Suiei) | [24,25,26,27,28,29] |
| Madagascar | Kotika (Antakarana); atsikana, fantsikakoholahy, patikakoho, teto, voafotsikotikana (Malagasy) | [30] |
| Mali (Dogonland) (Niono district) | Jene, surukujè (Bambara); nginnin (Malinké); jene (Minyanka); jeme (Sénoufo); sutèmi, sudèrèmi (Bwa); sumenh (Bobo-fing); segele (Dogon); déné, nkoloningé, baram baram, karam karam (Bambara); kolonidiè (Bamanan); ndjene (Bambara); segedere, ségélé, sèsègèrè, balambalam | [31,32,33,34,35] |
| Mozambique (Massingir district) | Nsangasi, sangasi (Changana) | [36] |
| Niger | Fulasco (Hausa), kamal (Peuhl), dagkirto (Béribéri), tsa | [37] |
| Nigeria | Iranje (Yoruba), tsuwawun karee (Hausa), njisinta (igbo) | [38,39] |
| Nigeria, Benin, Togo | Iranje (Yoruba people) | (a) |
| Philippines | Anislag or anislog, (Bikol, Cebu Bisaya, Samar-Leyte Bisaya, Manobo, Mandaya); anislang, hamislag (Bikol); katamangan, malangau (Manobo); tras (Magindanao) | [1] |
| Samoa | Poumuli | [1] |
| Senegal (Saloum Islands) | Sambelgorel, tembelforel (Peul); baram baram, dene, balamanten, tene (Bambara); mbarambaram, faragfarag, maymayin (Sérère); keng (Wolof); fusabel fu nene, e buker, ba herer (Diola); bouroum baran (Socé); tembel gorel (Peul, Toucouleur) | [33,40,41] |
| Solomon Islands | Mamufu’a or Mamufua (Kwara’ae), mewana (Bougainville Island) | [1] |
| Somalia | Xararaay | [42] |
| South Africa (Northern Maputaland), (Vhavenda), (Limpopo province), (Mpumalanga province), (Limpopo province) | Ihlalanyosi (Zulu); mutangauma (Venda); witbessiesbos (Afr.); mutangahuma (Tshivenda); mpfalambati (Tsonga); muhlakaume (Sotho); motlhalabu, motlhakawume, mohlakauma | [43,44,45,46] |
| Tanzania, Kenya, Mozambique (Swahili) | Mkwamba, mkwamba maji, mteja | [47] |
| Tanzania (Katoro Ward, Bukoba District), (Lushoto and Korogwe districts), (Handeni district), (Tanga district), (Kimboza forest reserve in Morogoro), (Muleba district) | Mkwambikwamb, lukwambikwambi (Kibunga); omubwera, omutoruka, muumbiti, muhumba, mkwamba, mtulavuha, msokote, mkalananga, mturuka, eruati, mkwambe (Ndengereko, Swahili, Zaramo) | [48,49,50,51,52] |
| Togo (Maritime region) | Kébantchalé, kébantialé, kégbantilé, akassélem, hesré (Mina, Ewé); tchakatchaka (Tem, Kabiyé, Fè, Adélé); tchacatchaca (Tem); hesre | [53] |
| Uganda (Budiope county), (Sango bay area), (Apac district), (Mabira Central Forest Reserve), (Langi language, Erute county, Lira district) | Lukandwa, olukandwa, iakara, ilakara, omukarara (Runyaruguru); omukalali (Rukonjo); lukandwa (Luganda) | [54,55,56,57,58,59,60] |
| Vanuatu | Mamau (Butu), nemameiu (Hiu) | [1] |
| Wallis and Futuna | Poutea | [1] |
| Zimbabwe (Harare and Nhema area, south-central region) | Muchagawuwe (Shona), umhakawuwe (Ndebele) | [61,62] |
| Zambia (Lusaka) | Lukuswaula | [1] |
|
|
|
|
|
|
|
|
|
|
|
|
| Compounds | Plant Parts Used (Location of Plant Collection) | Studies and Properties | References |
|---|---|---|---|
| Fluevirosinines A–D | Twigs and leaves (Guangxi, China) | Isolation, structural characterization, proposed biosynthetic pathway. Dimers, trimer, tetramer. | [182] |
| Fluevirosinines B–J | Twigs and leaves (Guangxi, China) | Isolation, structural characterization, proposed biosynthetic pathway. Inhibitory effect of fluevirosinine B on HIV-1 infected MT4 cells (EC50 = 14.1 μM), without cytotoxicity. Tetramers and pentamers. | [124] |
| Fluevirosines A–C | Twigs and leaves (China) | Isolation, structural characterization, biosynthetic pathway. No antiproliferative activity, but a weak inhibition (35% at 20 µM) of the splicing activity of XBP1 mRNA. | [184] |
| Fluevirosine D | Twigs and leaves (Guangxi, China) | [182] | |
| Fluevirines A–D | Twigs and leaves (China) | Isolation, structural characterization. Activity against Staphylococcus aureus. Fluevirine A showed activity against (MIC = 15.3 µg/mL). | [185] |
| Fluevirines E and F | Twigs and leaves (Xishuangbanna, Yunnan, China) | Very modest inhibition of cancer cell proliferation (<40% inhibition with fluevirine F at 40 µM). | [186] |
| Flueindolines A–C | Ripe fruit (Guangxi, China) | Isolation, structural characterization. Tested for neuroprotective activities on Neuro-2a cells and antivirus effects against the respiratory syncytial virus (RSV), but was found inactive. | [187] |
| Flueggines A and B | Twigs and leaves (China) | Isolation, structural characterization. Potent inhibition of cancer cell proliferation. Flueggine B strongly inhibited growth of MCF-7 and MDA-MB-231 cells (IC50 = 135 and 147 nM, respectively), whereas flueggine B was much less active. Total synthesis of flueggine A. | [188,189] |
| Flueggenines A and B | Roots (China) | Weak antiproliferative activity of flueggenine A against P388 leukemia cells (IC50 = 51.5 µM). | [190] |
| Flueggenines C and D | Twigs and leaves (Guangxi, China) | Isolation, structural characterization. Flueggenine D inhibited HIV-1 infection of MT4 cells (EC50 = 7.8 μM) and was weakly cytotoxic (selectivity index = 12.6). | [123,182] |
| Flueggenines E–I | Twigs and leaves (Xishuangbanna, Yunnan, China) | Isolation, structural characterization. Mild inhibition of MT4 cell infection by HIV-1 (EC50 = 42.6 μM) with flueggenine E. Enantioselective synthesis of flueggenines D and I. | [123,191] |
| Flueggedine | Twigs and leaves (China) | Isolation, structural characterization, proposed biosynthetic pathway from virosecurinine. No antiproliferative activity. | [183] |
| Flueggether A Virosinine A | Stems and leaves (China) | Isolation, structural characterization. Mild inhibitory effect on HIV-1-infected MT4 cells (EC50 = 120 and 45 μM, respectively) without cytotoxicity. | [6] |
| Flueggethers B–D | Twigs and leaves (Guangxi, China) | Isolation, structural characterization. Dimers (B–D), trimer (D). Inactive against tyrosine kinases c-Met and FGFR1. Flueggether D showed mild anti-HIV activity (CC50 = 43.1 μM) on MT-4 cells and was not cytotoxic (IC50 > 100 μM). | [192] |
| Fluvirosaones A and B | Twigs and leaves (China) | Isolation, structural characterization, biosynthetic pathway. Lipid-lowering effects in 3T3-L1 cells: weaker inhibition of triglyceride cellular accumulation compared with virosecurinine. | [193] |
| Bubbialine, Bubbialidine | Roots (Hainan, China) | Isolation. Total synthesis of bubbialidine. | [194,195] |
| Securinine, Norsecurinine | All plant parts | Total synthesis. Crystalline structure. | [196,197,198] |
| Securinol A Episecurinol A Secu’amamine E | Twigs and leaves (Guangxi, China) | Isolation, structural characterization. Total synthesis of secu’amamine E (and bubbialine). | [199,200] |
| Norsecurinine Norsecurinic acid Niruroidine | Roots (Hainan, China) | Isolation, structural characterization. (-)-Norsecurinine was found inactive against cancer cells and several bacteria. Total synthesis. | [194,201] |
| Norsecurinamines A and B | Fruit (China) | Isolation, structural characterization, proposed biosynthetic pathway from tryptophane. | [202] |
| Epoxynorsecurinine | Bark (Venda, South Africa) | Isolation, structural characterization. | [203] |
| ent-phyllanthidine ((+)-phyllanthidine) | Aerial parts (Saudi Arabia) | Quantification from a methanolic extract (biomarker). | [149] |
| Virosecurinine Viroallosecurinine | Leaves (Taiwan) | Isolation, structural characterization. Cytotoxic activity against cancer cell lines (EC50 = 3–8 μM). | [204] |
| Virosines A and B | Twigs and leaves (Guangdong, China) | Isolation, structural characterization. The two products and their stereoisomers. | [199,205] |
| Virosaines A and B | Twigs and leaves (China) | Isolation, structural characterization. Inactive against different cancer cell lines. Total synthesis of virosaines A and B. | [189,195,206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C. Traditional Uses, Pharmacology and Phytochemistry of the Medicinal Plant Flueggea virosa (Roxb. ex Willd.) Royle. Future Pharmacol. 2024, 4, 77-102. https://doi.org/10.3390/futurepharmacol4010007
Bailly C. Traditional Uses, Pharmacology and Phytochemistry of the Medicinal Plant Flueggea virosa (Roxb. ex Willd.) Royle. Future Pharmacology. 2024; 4(1):77-102. https://doi.org/10.3390/futurepharmacol4010007
Chicago/Turabian StyleBailly, Christian. 2024. "Traditional Uses, Pharmacology and Phytochemistry of the Medicinal Plant Flueggea virosa (Roxb. ex Willd.) Royle" Future Pharmacology 4, no. 1: 77-102. https://doi.org/10.3390/futurepharmacol4010007