Phytochemical Profiles and Antioxidant Activity of Grasses Used in South African Traditional Medicine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Evaluation
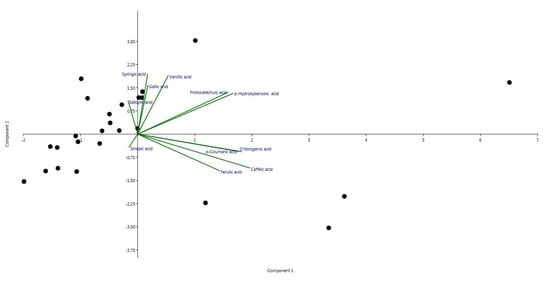
Concentration of Phenolic Acids
2.2. Antioxidant Activity
2.2.1. DPPH Radical-Scavenging Activity
2.2.2. Iron-Reducing Power
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extract Preparation
3.3. Phytochemical Evaluation
3.3.1. Quantification of Total Soluble Phenolic Content
3.3.2. Quantification of Flavonoid Content
3.3.3. Quantification of Iridoid Content
3.3.4. Quantification of Condensed Tannins
3.4. Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry (UHPLC–MS/MS)-Based Phenolic Acid Analysis
Instrumentation and Conditions
3.5. Determination of Antioxidant Activity
3.5.1. 1-Diphenyl-2-picrylhydrazyl (DPPH) Radical-Scavenging Activity
3.5.2. Ferric-Reducing-Power Assay
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Grasses Used in South African Traditional Medicine and Evaluated in the Current Study







References
- Palombo, E.A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: Modes of action and effects on intestinal function. Phytother. Res. 2006, 20, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Coley, P.D. Interspecific variation in plant anti-herbivore properties: The role of habitat quality and rate of disturbance. New Phytol. 1987, 106, 251–263. [Google Scholar] [CrossRef]
- Avoseh, O.; Oyedeji, O.; Rungqu, P.; Nkeh-Chungag, B.; Oyedeji, A. Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules 2015, 20, 7438–7453. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Ford, J.; Gaoue, O.G. Alkaloid-Poor Plant Families, Poaceae and Cyperaceae, Are Over-Utilized for Medicine in Hawaiian Pharmacopoeia. Econ. Bot. 2017, 71, 123–132. [Google Scholar] [CrossRef]
- Moerman, D.E. An analysis of the food plants and drug plants of native North America. J. Ethnopharmacol. 1996, 52, 1–22. [Google Scholar] [CrossRef]
- Stepp, J.R.; Moerman, D.E. The importance of weeds in ethnopharmacology. J. Ethnopharmacol. 2001, 75, 19–23. [Google Scholar] [CrossRef]
- Gebashe, F.; Aremu, A.; Finnie, J.; Van Staden, J. Grasses in South African traditional medicine: A review of their biological activities and phytochemical content. S. Afr. J. Bot. 2019, 122, 301–329. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-C.; Ng, L.T.; Chiang, W.; Chang, M.-Y.; Lin, C.-C. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 2003, 69, 600–604. [Google Scholar] [PubMed] [Green Version]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.M.; Hammouda, F.; Ismail, S.; Kamel, A.; Rimpler, H. Constituents of plants growing in Qatar part xxvii: Flavonoids of Cymbopogon parkeri. Qatar Univ. Sci. J. 1995, 15, 33–35. [Google Scholar]
- Cheel, J.; Theoduloz, C.; Rodríguez, J.; Schmeda-Hirschmann, G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.). J. Agric. Food Chem. 2005, 53, 2511–2517. [Google Scholar] [CrossRef]
- Chesselet, P.; Wolfson, M.M.; Ellis, R. A comparative histochemical study of plant polyphenols in southern African grasses. Afr. J. Range For. Sci. 1992, 9, 119–125. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kiderlen, A.F. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells. Phytochemistry 2005, 66, 2056–2071. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Gebashe, F.; Moyo, M.; Aremu, A.; Finnie, J.; Van Staden, J. Ethnobotanical survey and antibacterial screening of medicinal grasses in KwaZulu-Natal Province, South Africa. S. Afr. J. Bot. 2019, 22, 467–474. [Google Scholar] [CrossRef]
- Hartley, R.D.; Morrison, W.H. Monomeric and dimeric phenolic acids released from cell walls of grasses by sequential treatment with sodium hydroxide. J. Sci. Food Agric. 1991, 55, 365–375. [Google Scholar] [CrossRef]
- Kroon, P.A.; Williamson, G. Hydroxycinnamates in plants and food: Current and future perspectives. J. Sci. Food Agric. 1999, 79, 355–361. [Google Scholar] [CrossRef]
- Ou, S.; Li, Y.; Gao, K. A study on scavenging activity of wheat bran dietary fiber for free radical. Acta Nutr. Sin. 1999, 21, 191–194. [Google Scholar]
- Zhang, Z.; Yao, S.; Lin, W.; Wang, W.; Jin, Y.; Lin, N. Mechanism of reaction of nitrogen dioxide radical with hydroxycinnamic acid derivatives: A pulse radiolysis study. Free Radic. Res. 1998, 29, 13–16. [Google Scholar]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cuvelier, M.-E.; Richard, H.; Berset, C. Comparison of the antioxidative activity of some acid-phenols: Structure-activity relationship. Biosci. Biotechnol. Biochem. 1992, 56, 324–325. [Google Scholar] [CrossRef] [Green Version]
- Gülçin, I. Antioxidant Activity of Food Constituents: An Overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Shahidi, F.; Janitha, P.; Wanasundara, P. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Gerber, M.; Boutron-Ruault, M.-C.; Hercberg, S.; Riboli, E.; Scalbert, A.; Siess, M.-H. Food and cancer: State of the art about the protective effect of fruits and vegetables. Bull. Cancer 2002, 89, 293–312. [Google Scholar] [PubMed]
- Matteo, V.; Esposito, E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. CNS Neurol. Disord. Drug Targets 2003, 2, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Sreejayan, N.; Rao, M. Free radical scavenging activity of Curcuminoids. Arzneimittelforschung 1996, 46, 169–171. [Google Scholar] [PubMed]
- Gbenou, J.D.; Ahounou, J.F.; Akakpo, H.B.; Laleye, A.; Yayi, E.; Gbaguidi, F.; Baba-Moussa, L.; Darboux, R.; Dansou, P.; Moudachirou, M. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol. Biol. Rep. 2013, 40, 1127–1134. [Google Scholar] [CrossRef]
- Soares, M.O.; Alves, R.C.; Pires, P.C.; Oliveira, M.B.P.; Vinha, A.F. Angolan Cymbopogon citratus used for therapeutic benefits: Nutritional composition and influence of solvents in phytochemicals content and antioxidant activity of leaf extracts. Food Chem. Toxicol. 2013, 60, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Aremu, A.O.; Amoo, S.O.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Antioxidant activity, acetylcholinesterase inhibition, iridoid content and mutagenic evaluation of Leucosidea sericea. Food Chem. Toxicol. 2011, 49, 1122–1128. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree Foliage. A Laboratory Manual for the FAO/IAEA Coordinated Research Project on ‘Use of Nuclear and Related Techniques to Develop Simple Tannin Assay for Predicting and Improving the Safety and Efficiency of Feeding Ruminants on the Tanniniferous Tree Foliage’; Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture: Vienna, Austria, 1999; pp. 1–29. [Google Scholar]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Levieille, G.; Wilson, G. In vitro propagation and iridoid analysis of the medicinal species Harpagophytum procumbens and H. zeyheri. Plant Cell Rep. 2002, 21, 220–225. [Google Scholar]
- Haag-Berrurier, M.; Kuballa, B.; Anton, R. Dosage des glucoiridoïdes totaux dans la racine d’Harpagophytum procumbens DC. Plant Med. Phytother. 1978, 12, 197–206. [Google Scholar]
- Gruz, J.; Novák, O.; Strnad, M. Rapid analysis of phenolic acids in beverages by UPLC–MS/MS. Food Chem. 2008, 111, 789–794. [Google Scholar] [CrossRef]
- Karioti, A.; Hadjipavlou-Litina, D.; Mensah, M.L.; Fleischer, T.C.; Skaltsa, H. Composition and antioxidant activity of the essential oils of Xylopia aethiopica (Dun) A. Rich.(Annonaceae) leaves, stem bark, root bark, and fresh and dried fruits, growing in Ghana. J. Agric. Food Chem. 2004, 52, 8094–8098. [Google Scholar] [CrossRef]
- Lim, T.; Lim, Y.; Yule, C. Evaluation of antioxidant, antibacterial and anti-tyrosinase activities of four Macaranga species. Food Chem. 2009, 114, 594–599. [Google Scholar] [CrossRef]




| Plant Species | Plant Part | Hydroxybenzoic Acids (µg/g DW) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Phenolic Content (mg GAE/g DW) | Gallic Acid | p-Hydroxy-Benzoic Acid | Protocatechuic Acid | Salicylic Acid | Syringic Acid | Vanillic Acid | ||
| Coix lacryma-jobi | Roots | 9.0 ± 0.95 b,c | <LOD | 9.3 ± 1.032 d,e | 5.9 ± 0.39 b | 1.6 ± 0.14 m | 2.9 ± 0.52 e,f,g,h,i | 11.6 ± 0.34 j,k,l |
| Leaves | 12.2 ± 1.38 b | 0.4 ± 0.03 b,c | 34.2 ± 2.96 a | 9.0 ± 0.84 a | 1.8 ± 0.13 l,m | 3.1 ± 0.23 d,e,f,g,h | 34.4 ± 0.24 b | |
| Cenchrus ciliaris | Roots | 26.6 ± 1.99 a | 0.2 ± 0.04 d | 10.3 ± 0.21 c,d | 0.6 ± 0.08 i | 67.4 ± 2.34 e | 3.6 ± 0.11 c,d | 14.8 ± 0.58 h,i,j |
| Leaves | 9.0 ± 0.93 b,c | 0.5 ± 0.13 b | 10.7 ± 0.38 c,d | 1.2 ± 0.04 f,g,h,i | 248.2 ± 3.875 c | 5.4 ± 0.09 b | 16.9 ± 0.58 f,g,h,i | |
| Cynodon dactylon | Whole plant | 6.3 ± 0.32 c–f | 0.1 ± 0.00 e,f | 3.3 ± 0.18 | 0.7 ± 0.03 h,i | 37.8 ± 1.06 g | 1.5 ± 0.08 l | 12.6 ± 0.45 j,k,l |
| Cymbopogon nardus | Roots | 28.9 ± 1.81 a | 0.1 ± 0.00 e,f | 9.2 ± 0.18 d,e | 3.8 ± 0.06 c | 8.2 ± 0.22 k,l,m | 2.4 ± 0.10 i,j,k | 14.1 ± 0.45 i,j,k |
| Leaves | 9.1 ± 0.90 b–d | 0.1 ± 0.02 e,f | 6.9 ± 0.16 f,g | 2.5 ± 0.09 c,d,e,f,g | 5.3 ± 0.16 k,l,m | 5.0 ± 0.24 b | 19.6 ± 1.24 d,e,f | |
| Inflorescence | 8.9 ± 0.84 b,c | 0.3 ± 0.13 c | 11.4 ± 0.49 c | 6.4 ± 0.16 b | 2.4 ± 0.10 l,m | 3.9 ± 0.18 c | 18.4 ± 1.49 d,e,f,g | |
| Cymbopogon spp. | Roots | 30.9 ± 2.93 a | 0.1 ± 0.01 e,f | 68 ± 0.76 f,g | 2.0 ± 0.22 d,e,f,g,h,i | 13.2 ± 0.54 j,k | 2.9 ± 0.33 e,f,g,h,i | 11.1 ± 0.93 k,l |
| Leaves | 9.8 ± 0.38 b,c | 0.8 ± 0.21 a | 6.7 ± 0.15 f,g | 2.9 ± 0.13 c,d,e | 4.6 ± 0.07 k,l,m | 2.7 ± 0.20 f,g,h,i,j | 18.5 ± 0.51 d,e,f,g | |
| Eragrostis curvula | Roots | 5.3 ± 0.13 d–f | 0.1 ± 0.02 e,f | 9.8 ± 0.34 c,d | 1.8 ± 0.09 d,e,f,g,h,i | 26.7 ± 0.42 h,i | 3.3 ± 0.37 d,e,f,g | 46.05 ± 2.38 a |
| Leaves | 7.4 ± 0.53 b–d | 0.2 ± 0.03 d | 5.3 ± 0.55 g | 2.1 ± 0.13 d,e,f,g,h | 30.5 ± 1.33 g,h | 3.3 ± 0.40 c,d,e,f,g | 21.7 ± 1.3 c,d | |
| Inflorescence | - | 0.1 ± 0.00 e,f | 10.7 ± 0.79 c,d | 9.0 ± 0.38 a | 7.3 ± 0.18 k,l,m | 7.0 ± 0.53 a | 36.0 ± 1.12 b | |
| Imperata cylindrica | Roots | 6.4 ± 0.49 c–f | 0.3 ± 0.06 c | 6.9 ± 0.19 f,g | 3.1 ± 0.13 c,d | 12.1 ± 0.49 j,k,l | 3.4 ± 0.27 c,d,e | 24.4 ± 1.24 c |
| Leaves | 7.3 ± 0.14 b–d | 0.2 ± 0.01 d,e | 6.834 ± 0.436 f,g | 3.1 ± 0.07 c,d | 19.3 ± 0.77 i,j | 2.7 ± 0.12 g,h,i,j | 24.0 ± 1.45 c | |
| Panicum maximum | Roots | 6.9 ± 0.24 c,d | 0.1 ± 0.01 e,f | 9.9 ± 0.43 c,d | 2.9 ± 0.10 c,d,e | 47.6 ± 2.63 f | 3.2 ± 0.09 d,e,f,g,h | 21.4 ± 0.95 c,d,e |
| Leaves | 9.8 ± 1.00 b,c | 0.3 ± 0.02 c,d | 10.26 ± 0.197 c,d | 6.9 ± 2.70 b | 356.82 ± 12.92 b | 3.1 ± 0.19 d,e,f,g,h | 14.6 ± 0.41 h,i,j,k | |
| Setaria megaphylla | Roots | 8.0 ± 0.49 b–d | 0.1 ± 0.01 e,f | 11.2 ± 1.32 c | 1.6 ± 0.29 e,f,g,h,i | 93.1 ± 6.12 d | 3.4 ± 0.56 c,d,e | 23.5 ± 4.77 c |
| Leaves | 8.6 ± 0.62 b–d | 0.2 ± 0.021 d,e | 14.0 ± 1.55 b | 2.5 ± 0.26 c,d,e,f | 467.3 ± 15.36 a | 3.3 ± 0.35 c,d,e,f | 21.1 ± 3.67 c,d,e | |
| Sporobolus africanus | Roots | 4.2 ± 0.21 f | 0.1 ± 0.01 e,f | 6.0 ± 0.29 f,g | 1.9 ± 0.04 d,e,f,g,h,i | 2.9 ± 0.05 k,l,m | 2.2 ± 0.17 j,k | 10.4 ± 0.41 l |
| Leaves | 6.7 ± 0.45 c–f | 0.1 ± 0.05 e,f | 6.0 ± 0.15 f,g | 1.1 ± 0.08 g,h,i | 5.2 ± 0.10 k,l,m | 2.0 ± 0.15 k,l | 12.5 ± 0.29 j,k,l | |
| Sporobolus pyramidalis | Roots | 4.4 ± 0.45 e,f | <LOD | 6.2 ± 0.22 f,g | 1.2 ± 0.13 f,g,h,i | 5.3 ± 0.19 k,l,m | 3.4 ± 0.28 c,d,e | 20.8 ± 1.84 c,d,e |
| Leaves | 6.4 ± 0.29 c–f | 0.2 ± 0.06 d,e | 7.7 ± 0.23 e,f | 1.5 ± 0.10 f,g,h,i | 4.7 ± 0.04 k,l,m | 2.5 ± 0.06 h,i,j,k | 16.7 ± 1.24 f,g,h,i | |
| Vetiveria zizanioides | Roots | 7.3 ± 0.28 b–d | 0.1 ± 0.01 e,f | 3.5 ± 0.15 h | 1.4 ± 0.01 f,g,h,i | 2.6 ± 0.06 l,m | 3.3 ± 0.20 c,d,e,f | 15.1 ± 0.86 g,h,i,j |
| Leaves | 6.0 ± 0.46 c–f | 0.1 ± 0.010 e,f | 7.2 ± 0.18 f,g | 1.8 ± 0.09 d,e,f,g,h,i | 4.6 ± 0.13 k,l,m | 3.6 ± 0.14 c,d | 17.8 ± 0.58 e,f,g,h | |
| Plant Species | Plant Part | Hydroxycinnamic Acids (µg/g DW) | |||||
|---|---|---|---|---|---|---|---|
| Total Phenolic Content (mg GAE/g DW) | Caffeic Acid | Chlorogenic Acid | p-Coumaric Acid | Ferulic Acid | Sinapic Acid | ||
| Coix lacryma-jobi | Roots | 9.0 ± 0.95 b,c | 14.1 ± 1.39 c | 144.8 ± 11.28 a | 59.8 ± 7.31 b | 22.9 ± 5.28 d | <LOD |
| Leaves | 12.2 ± 1.38 b | 16.3 ± 1.50 b | 105.3 ± 8.50 b | 80.4 ± 5.45 a | 26.8 ± 2.31 c | 0.3 ± 0.03 m | |
| Cenchrus ciliaris | Roots | 26.6 ± 1.99 a | 0.3 ± 0.03 i,j | <LOD | 34.6 ± 2.44 e,f | 9.4 ± 0.57 h,i,j | 0.9 ± 0.04 f,g |
| Leaves | 9.0 ± 0.93 b,c | 0.4 ± 0.04 i,j | <LOD | 32.2 ± 0.51 e,f | 9.0 ± 0.21 h,i,j | 2.4 ± 0.07 a | |
| Cynodon dactylon | Whole plant | 6.3 ± 0.32 c–f | 0.1 ± 0.02 j | 0.2 ± 0.02 f | 23.1 ± 0.74 h,i | 7.5 ± 0.23 j,k | 0.6 ± 0.03 i,j,k |
| Cymbopogon nardus | Roots | 28.9 ± 1.81 a | 18.0 ± 0.24 a | 35.5 ± 1.20 f | 64.0 ± 0.33 b | 56.0 ± 0.74 a | 2.1 ± 0.015 c |
| Leaves | 9.1 ± 0.90 b–d | 3.6 ± 0.64 f | 4.6 ± 0.64 f | 51.4 ± 0.66 c | 14.3 ± 1.48 f,g | 0.6 ± 0.03 i,j,k | |
| Inflorescence | 8.9 ± 0.84 b,c | 1.1 ± 0.07 h,i,j | 0.1 ± 0.01 f | 24.2 ± 0.85 g,h,i | 15.3 ± 0.37 f | 0.9 ± 0.03 f | |
| Cymbopogon spp. | Roots | 30.9 ± 2.93 a | 10.8 ± 1.11 d | 12.3 ± 1.70 d,e | 60.3 ± 3.45 b | 33.1 ± 1.98 b | 1.6 ± 0.18 d |
| Leaves | 9.8 ± 0.38 b–e | 1.4 ± 0.05 g,h,i | 3.6 ± 0.71 f | 25.5 ± 0.73 g,h | 8.0 ± 0.22 i,j,k | 0.4 ± 0.02 l,m | |
| Eragrostis curvula | Roots | 5.3 ± 0.13 d–f | 1.5 ± 0.132 g,h,i | 0.3 ± 0.01 f | 19.2 ± 0.69 i,j | 24.4 ± 0.90 c,d | 1.2 ± 0.14 e |
| Leaves | 7.4 ± 0.53 b–d | 1.3 ± 0.11 g,h,i,j | 3.3 ± 0.26 f | 22.0 ± 1.69 h,i,j | 17.1 ± 1.40 e,f | 1.4 ± 0.18 d | |
| Inflorescence | - | 4.1 ± 0.44 f | 0.7 ± 0.02 f | 16.6 ± 0.40 j,k | 21.7 ± 1.58 d | 0.8 ± 0.08 f,g,h | |
| Imperata cylindrica | Roots | 6.4 ± 0.49 c–f | 1.5 ± 0.12 g,h,i | 15.3 ± 1.00 d | 32.0 ± 1.89 e,f | 9.7 ± 0.43 h,i,j | 1.0 ± 0.09 e,f |
| Leaves | 7.3 ± 0.14 b–d | 1.9 ± 0.15 g,h | 14.2 ± 0.58 d | 29.2 ± 1.01 f,g | 8.8 ± 0.51 h,i,j,k | 0.5 ± 0.01 j,k,l,m | |
| Panicum maximum | Roots | 6.9 ± 0.24 c,d | 0.7 ± 0.01 i,j | 1.8 ± 0.17 f | 46.3 ± 1.19 c,d | 10.6 ± 0.33 h,i,j | 0.7 ± 0.03 g,h,i |
| Leaves | 9.8 ± 1.00 b,c | 2.3 ± 0.09 g | 3.0 ± 0.15 f | 35.5 ± 1.94 e | 10.4 ± 0.14 h,i,j | 0.5 ± 0.01 k,l,m | |
| Setaria megaphylla | Roots | 8.0 ± 0.49 b–d | 0.5 ± 0.07 i,j | 1.9 ± 0.37 f | 44.7 ± 4.14 d | 9.7 ± 1.18 h,i,j | 0.6 ± 0.08 i,j,k |
| Leaves | 8.6 ± 0.62 b–d | 2.5 ± 0.46 g | 6.9 ± 0.48 e,f | 42.8 ± 4.70 d | 15.5 ± 1.45 f | 0.6 ± 0.09 i,j,k | |
| Sporobolus africanus | Roots | 4.2 ± 0.21 f | 6.4 ± 0.48 e | 0.1 ± 0.01 f | 10.4 ± 0.88 l | 5.7 ± 0.30 k | 0.3 ± 0.05 l,m |
| Leaves | 6.7 ± 0.45 c–f | 0.6 ± 0.03 i,j | 0.1 ± 0.00 f | 20.1 ± 0.76 h,i,j | 11.7 ± 0.21 g,h | 0.7 ± 0.03 h,i,j | |
| Sporobolus pyramidalis | Roots | 4.4 ± 0.45 e,f | 0.5 ± 0.07 i,j | < LOD | 11.0 ± 0.60 l | 18.7 ± 0.30 e | 0.4 ± 0.11 l,m |
| Leaves | 6.4 ± 0.29 c–f | 0.6 ± 0.05 i,j | < LOD | 12.2 ± 0.41 k,l | 11.2 ± 0.44 h,i | 0.5 ± 0.06 i,j,k,l | |
| Vetiveria zizanioides | Roots | 7.3 ± 0.28 b–d | 1.4 ± 0.04 g,h,i | 1.6 ± 0.29 f | 30.8 ± 0.33 e,f | 22.1 ± 0.71 d | 2.2 ± 0.177 b |
| Leaves | 6.0 ± 0.46 c–f | 0.8 ± 0.05 h,i,j | 2.1 ± 0.13 f | 46.3 ± 0.50 c,d | 10.7 ± 0.25 h,i,j | 0.4 ± 0.05 l,m | |
| Grass Species | Plant Part | EC50 (mg/mL) |
|---|---|---|
| Coix lacryma-jobi | Roots | 0.09 ± 0.017 a,b,c,d,e |
| Leaves | 0.10 ± 0.004 a,b,c | |
| Cenchrus ciliaris | Roots | 0.09 ± 0.006 a,b,c,d,e |
| Leaves | 0.09 ± 0.011 a,b,c,d,e | |
| Cynodon dactylon | Whole plant | 0.10 ± 0.005 a,b,c,d |
| Cymbopogon nardus | Roots | 0.02 ± 0.007 h |
| Leaves | 0.06 ± 0.002 f,g | |
| Inflorescence | 0.04 ± 0.012 f,g | |
| Cymbopogon spp. | Roots | 0.04 ± 0.026 g,h |
| Leaves | 0.08 ± 0.017 d,e,f | |
| Eragrostis curvula | Roots | 0.11 ± 0.012 a,b |
| Leaves | 0.08± 0.019 c,d,e,f | |
| Imperata cylindrica | Roots | 0.10 ± 0.000 a,b,c,d,e |
| Leaves | 0.09 ± 0.001 a,b,c,d,e | |
| Panicum maximum | Roots | 0.11 ± 0.021 a,b,c |
| Leaves | 0.08 ± 0.011 b,d,e,f | |
| Setaria megaphylla | Roots | 0.11 ± 0.017 a |
| Leaves | 0.07 ± 0.005 e,f | |
| Sporobolus africanus | Roots | 0.10 ± 0.010 a,b,c,d |
| Leaves | 0.10 ± 0.002 a,b,c,d | |
| Sporobolus pyramidalis | Roots | 0.08 ± 0.014 b,d,e,f |
| Leaves | 0.08 ± 0.001 b,d,e,f | |
| Vetiveria zizanioides | Roots | 0.11 ± 0.004 a,b,c |
| Leaves | 0.06 ± 0.014 f,g |
| Grass Species | Plant Part | Slope | R2 |
|---|---|---|---|
| Coix lacryma-jobi | Roots | 0.054 ± 0.003 | 0.6896 |
| Leaves | 0.060 ± 0.002 | 0.6723 | |
| Cenchrus ciliaris | Roots | 0.071 ± 0.002 | 0.7935 |
| Leaves | 0.069 ± 0.0032 | 0.7817 | |
| Cynodon dactylon | whole plant | 0.078 ± 0.001 | 0.8988 |
| Cymbopogon nardus | Roots | 0.070 ± 0.005 | 0.5142 |
| Leaves | 0.079 ± 0.014 | 0.6405 | |
| Inflorescence | 0.074 ± 0.002 | 0.6545 | |
| Cymbopogon spp. | Roots | 0.069 ± 0.003 | 0.6317 |
| Leaves | 0.082 ± 0.007 | 0.7303 | |
| Eragrostis curvula | Roots | 0.070 ± 0.004 | 0.7879 |
| Leaves | 0.051 ± 0.005 | 0.6267 | |
| Imperata cylindrica | Roots | 0.079 ± 0.005 | 0.7213 |
| Leaves | 0.062 ± 0.009 | 0.6726 | |
| Panicum maximum | Roots | 0.076 ± 0.003 | 0.8495 |
| Leaves | 0.077 ± 0.001 | 0.7304 | |
| Setaria megaphylla | Roots | 0.073 ± 0.002 | 0.7927 |
| Leaves | 0.059 ± 0.001 | 0.6646 | |
| Sporobolus africanus | Roots | 0.124 ± 0.004 | 0.8667 |
| Leaves | 0.086 ± 0.001 | 0.8547 | |
| Sporobolus pyramidalis | Roots | 0.094 ± 0.003 | 0.8509 |
| Leaves | 0.081 ± 0.003 | 0.6991 | |
| Vetiveria zizanioides | Roots | 0.071 ± 0.007 | 0.825 |
| Leaves | 0.069 ± 0.012 | 0.7591 | |
| Ascorbic acid (positive control) | 0.095 ± 0.001 | 0.999 | |
| Sequence | Solvent | Duration (min) |
|---|---|---|
| 1 | 5% B | 0.8 |
| 2 | 5%–10% B | 0.4 |
| 3 | Isocratic 10% B | 0.7 |
| 4 | 10%–15% B | 0.5 |
| 5 | Isocratic 15% B | 1.3 |
| 6 | 15%–21% B | 0.3 |
| 7 | Isocratic 21% B | 1.2 |
| 8 | 21%–27% B | 0.5 |
| 9 | 27%–50% B | 2.3 |
| 10 | 50%–100% B | 1.0 |
| 11 | 100%–5% | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebashe, F.; Aremu, A.O.; Gruz, J.; Finnie, J.F.; Van Staden, J. Phytochemical Profiles and Antioxidant Activity of Grasses Used in South African Traditional Medicine. Plants 2020, 9, 371. https://doi.org/10.3390/plants9030371
Gebashe F, Aremu AO, Gruz J, Finnie JF, Van Staden J. Phytochemical Profiles and Antioxidant Activity of Grasses Used in South African Traditional Medicine. Plants. 2020; 9(3):371. https://doi.org/10.3390/plants9030371
Chicago/Turabian StyleGebashe, Fikisiwe, Adeyemi O. Aremu, Jiri Gruz, Jeffrey F. Finnie, and Johannes Van Staden. 2020. "Phytochemical Profiles and Antioxidant Activity of Grasses Used in South African Traditional Medicine" Plants 9, no. 3: 371. https://doi.org/10.3390/plants9030371