Proximate Analyses and Amino Acid Composition of Selected Wild Indigenous Fruits of Southern Africa
Abstract
:1. Introduction
1.1. Carissa macrocarpa (Eckl.) A.DC. (Amathungulu, Big Num-Num)
1.2. Carpobrotus edulis (L.) N.E.Br. (Sour Fig, Hottentots Fig; Ghaukum, Ghoenavy, Hottentotsvy, Perdevy, Rankvy, Suurvy, Ikhambi-Lamabulawo, Umgongozi)
1.3. Cordyla africana Lour. (Sunbird Tree, Wildemango, iGowane-Elikhulu, Umbhone)
1.4. Dovyalis caffra (Hook.f. & Harv.) Warb. (Kei-Apple, Kei-Appel, Motlhono, Umqokolo, Amaqokolo, Mukokolo)
1.5. Dovyalis longispina Warb. (Coastal Kei-Apple, Natal Apricot)
1.6. Englerophytum magalismontanum (Sonder) T.D.Penn (Transvaal Milkplum, Stamvrug, Motlhatswa, Mohlatswa, Munombelo, Amanumbela, Umnumbela)
1.7. Garcinia livingstonei T.Anderson (African Mangosteen, Afrika-Geelmelkhout, umPhimbi, uGobandlovu, Mmimbi, Mokongono, Mokononga)
1.8. Halleria lucida L. (Tree Fuchsia, White Olive, Notsung, Witolienhout, Witolyfhout, Umbinza, Indomela, Umbinza, Lebetsa, Murevhe)
1.9. Manilkara mochisia (Baker) Dubard (Lowveld milkberry (English) Mwambo (Shona)
1.10. Pappea capensis Eckl. & Zeyh. (Jacket Plum, Indaba Tree, Bushveld Cherry, Doppruim, Umqhokwane, Umvuna, Indaba, Ilitye, Umgqalutye, Mongatane, Mopsinyugane, Liletsa, Xikwakwaxu, Gulaswimbi)
1.11. Parinari curaellifolia Planch ex. Benth. (Mobola Plum, Cork Tree, Hissing Tree, Grysappel, Bosappel, Mmola, Mobola, Muvhula)
1.12. Phoenix reclinata Jacq. (Wild Date Palm, Wilde-Dadelboom, Mopalamo, Moséfa, Isundu)
1.13. Syzygium cordatum Hochst. (Water Berry, Waterbessie, Waterbessieboom, Waterboom, Waterhout, Umdoni, Umswi, Umjomi, Mawthoo, Motlho, Mutu, Muhlwa)
1.14. Syzygium guineense DC. (Woodland Waterberry, Waterpear, Waterpeer, Umdoni)
2. Results and Discussion
2.1. Literature Survey
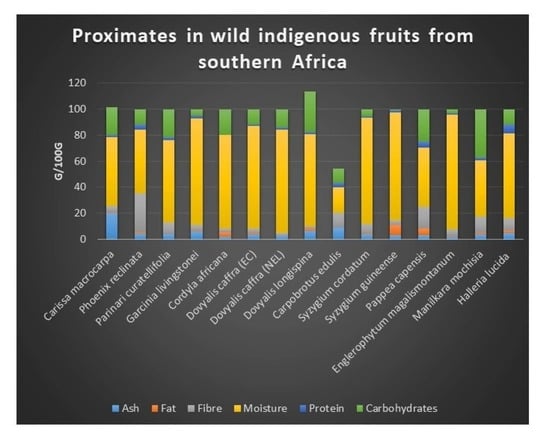
2.2. Proximate Composition
2.3. Amino Acid Composition
3. Materials and Methods
3.1. Literature Survey
3.2. Fruit Samples for Evaluation
3.2.1. Selected Fruits for Analysis
3.2.2. Processing of Fruits
3.3. Proximate Analysis
3.3.1. Moisture Content Determination
3.3.2. Protein Content Determination
3.3.3. Ash Content Determination
3.3.4. Fat Content Determination
3.3.5. Fiber Content Determination
3.3.6. Carbohydrates Determination
3.4. Amino Acid Analysis
3.5. Determination of Energy Value
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Calculation of the Energy Content of Foods-Energy Commission Factors; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Fanzo, J.; Hunter, D.; Borelli, T.; Mattei, F. Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health; Routledge: Abingdon-on-Thames, UK, 2013. [Google Scholar]
- Guled, R.A.; Nik, M.M.; Wan, A.M.; Abu, B.; Tefera, B.; Nega, A. Undernutrition prevalence and its determinants among children below five years of age in Shabelle Zone, Somali Region, Eastern Ethiopia. Intern. J. All. Health Sci. 2017, 1, 72–91. [Google Scholar]
- WHO. Nutrition in the WHO African Region; World Health Organization: Brazzaville, Congo, 2017. [Google Scholar]
- Duguma, H.F. Wild edible plant nutritional contribution and consumer perception in Ethiopia. Intern. J. Food Sci. 2020, 2958623. [Google Scholar] [CrossRef]
- Nieman, D.C.; Lee, R. Nutritional Assessment, 3rd ed.; McGraw-Hill: Boston, MA, USA, 2003. [Google Scholar]
- AOAC. Official Methods of Analysis Association of Official Analytical, Chemist, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Hart, F.L.; Fisher, H.J. Modern Food Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Saka, J.K.; Msonthi, J.D. Nutritional value of edible fruits of indigenous wild trees in Malawi. For. Ecol. Manag. 1994, 64, 245–248. [Google Scholar] [CrossRef]
- Galli, F. Amino acid and protein modification by oxygen and nitrogen species. Amino Acids 2007, 32, 497–499. [Google Scholar] [CrossRef]
- Ha, E.; Zeme, M.B. Functional properties of whey, whey components, and essential amino acids: Mechanisms underlying health benefits for active people. J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Carolus, B.; Porter, H.; Reynolds, Y. Carissa macrocarpa. 2004. Available online: http://pza.sanbi.org/carissa-macrocarpa (accessed on 14 February 2021).
- Pfukwa, T.M.; Chikwanha, O.C.; Katiyatiya, C.L.F.; Fawole, A.O.; Manley, M.; Mapiye, C. Southern African indigenous fruits and their by-products: Prospects as food antioxidants. J. Funct. Foods 2020, 75, 104220. [Google Scholar] [CrossRef]
- Tropical Plants Database, Ken Fern, Carissa macrocarpa. Available online: tropical.theferns.info/viewtropical.php?id=Carissa+macrocarpa (accessed on 14 February 2021).
- Van Wyk, B.-E. The potential of South African plants in the development of new food and beverage products. S. Afr. J. Bot. 2011, 77, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Tropical Plants Database, Ken Fern, Carpobrotus edulis. Available online: tropical.theferns.info/viewtropical.php?id=Carpobrotus+edulis (accessed on 14 February 2021).
- Malan, C.; Notten, A. Carpobrotus edulis. 2006. Available online: http://pza.sanbi.org/carpobrotus-edulis (accessed on 14 February 2021).
- Meyer, J. Cordyla africana. 2006. Available online: http://pza.sanbi.org/cordyla-africana (accessed on 22 February 2021).
- Lemmens, R.H.M.J.; Nyunaï, N. Cordyla africana Lour; Record from, PROTA4U; Lemmens, R.H.M.J., Louppe, D., Oteng-Amoako, A.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources Végétales de L’afrique Tropicale): Wageningen, The Netherlands, 2011. [Google Scholar]
- Ndou, P. Dovyalis caffra. 2003. Available online: http://pza.sanbi.org/dovyalis-caffra (accessed on 23 February 2021).
- Roux, J.P. Dovyalis caffra. In Flora of South Africa; South African Biodiversity Institute: Pretoria, South Africa, 2003; Available online: https://plants.jstor.org/compilation/dovyalis.caffra (accessed on 23 February 2021).
- Aremu, A.O.; Ncama, K.; Omotayo, A.O. Ethnobotanical uses, biological activities and chemical properties of Kei-apple [Dovyalis caffra (Hook.f. & Harv.) Sim]: An indigenous fruit tree of Southern Africa. J. Ethnopharmacol. 2019, 241, 111963. [Google Scholar] [CrossRef] [PubMed]
- Roux, J.P. Dovyalis longispina. In Flora of South Africa; South African Biodiversity Institute: Pretoria, South Africa, 2003; Available online: https://plants.jstor.org/compilation/Dovyalis.longispina (accessed on 23 February 2021).
- Behr, K. Englerophytum magalismontanum. 2004. Available online: http://pza.sanbi.org/englerophytum-magalismontanum (accessed on 24 February 2021).
- Becking, D. Englerophytum magalismontanum. Available online: https://treesa.org/englerophytum-magalismontanum/ (accessed on 24 February 2021).
- Rampedi, I.T. Indigenous Plants in the Limpopo Province: Potential for Their Commercial Beverage Production. Ph.D. Thesis, University of Limpopo, Polokwane, South Africa, 2010. [Google Scholar]
- Glen, H. Garcinia livingstonei. 2007. Available online: http://pza.sanbi.org/garcinia-livingstonei (accessed on 24 February 2021).
- Coates Palgrave, M. Trees of Southern Africa, 3rd ed.; Struik: Cape Town, South Africa, 2002. [Google Scholar]
- Tropical Plants Database, Ken Fern, Garcinia livingstonei. Available online: tropical.theferns.info/viewtropical.php?id=Garcinia+livingstonei (accessed on 3 February 2021).
- Kaikabo, A.A.; Samuel, B.B.; Eloff, J.N. Isolation and activity of two antibacterial biflavonoids from Garcinia livingstonei leaf extract. Nat. Prod. Commun. 2009, 4, 1363–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaikabo, A.A.; Eloff, J.N. Antibacterial activity of two biflavonoids from Garcinia livingstonei leaves against Mycobacterium smegmatis. J. Ethnopharmacol. 2011, 138, 253–255. [Google Scholar] [CrossRef] [Green Version]
- Mbabazeli, G.; Notten, A. Halleria lucida. 2018. Available online: http://pza.sanbi.org/halleria-lucida (accessed on 25 February 2021).
- Hemsley, J.A. Sapotaceae. In Flora of Tropical East Africa; Royal Botanic Gardens, Kew: Richmond, UK, 1968; p. 1. [Google Scholar]
- Tshikalange, E.; Modishane, D.C.; Tabit, F.T. Antimicrobial, antioxidant, and cytotoxicity properties of selected wild edible fruits of traditional medicinal plants. J. Herbs Spices Med. Plants 2017, 23, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Hankey, A. Pappea capensis. 2004. Available online: http://pza.sanbi.org/pappea-capensis (accessed on 27 February 2021).
- Pendota, S.C.; Aderogba, M.A.; Moyo, M.; McGaw, L.J.; Mulaudzi, R.B.; Van Staden, J. Antimicrobial, antioxidant and cytotoxicity of isolated compounds from leaves of Pappea capensis. S. Afr. J. Bot. 2017, 108, 272–277. [Google Scholar] [CrossRef]
- Maharaj, V.; Glen, H.F. Parinari curatellifolia. 2008. Available online: http://pza.sanbi.org/parinari-curatellifolia (accessed on 27 February 2021).
- Muchuweti, M.; Matongo, N.; Benhura, M.A.N.; Bhebhe, M.; Kasiyamhuru, A.; Chipurura, B. Nutritional composition of Parinari curatellifolia fruit and a jam made from the pulp of the fruit: An untapped resource. Acta Hortic. 2013, 979, 621–624. [Google Scholar] [CrossRef]
- Tropical Plants Database, Ken Fern, Phoenix reclinata. Available online: tropical.theferns.info/viewtropical.php?id=Phoenix+reclinata (accessed on 23 February 2021).
- Aubrey, A. Phoenix reclinata. 2004. Available online: http://pza.sanbi.org/phoenix-reclinata (accessed on 27 February 2021).
- Carolus, B. Syzygium cordatum. 2004. Available online: http://pza.sanbi.org/syzygium-cordatum (accessed on 27 February 2021).
- Maroyi, A. Syzygium cordatum Hochst. ex Krauss: An overview of its ethnobotany, phytochemistry and pharmacological properties. Molecules 2018, 23, 1084. [Google Scholar] [CrossRef] [Green Version]
- Zenze, K. Syzygium guineense. 2013. Available online: http://pza.sanbi.org/syzygium-guineense (accessed on 28 February 2021).
- Tropical Plants Database, Ken Fern, Syzygium guineense. Available online: tropical.theferns.info/viewtropical.php?id=Syzygium+guineense (accessed on 25 February 2021).
- Sibiya, N.P.; Kayitesi, E.; Moteetee, A. Mineral composition of selected Southern African fruits. S. Afr. J. Bot. 2020, 132, 87–94. [Google Scholar] [CrossRef]
- Aganga, A.A.; Mosase, K.W. Tannin content, nutritive value, and dry matter digestibility of Lonchocarpus capassa, Ziziphus mucronata, Sclerocarya birrea, Kirkia acuminata and Rhus lancea seeds. Anim. Feed Sci. Technol. 2001, 91, 7–113. [Google Scholar] [CrossRef]
- Eromosele, I.C.; Eromosele, C.O.; Kuzhkuzha, D.M. Evaluation of mineral elements and ascorbic acid contents in fruits of some wild plants. Plant Foods Hum. Nutr. 1991, 41, 151–154. [Google Scholar] [CrossRef]
- Jaenicke, H.; Thiong’o, M.K. Preliminary nutritional analysis of marula (Sclerocarya birrea) fruits from two Kenyan provenances. Acta Hortic. 2000, 531, 245–250. [Google Scholar] [CrossRef]
- Legwaila, G.M.; Mojeremane, W.; Madisa, M.E.; Mmolotsi, R.M.; Rampart, M. Potential of traditional food plants in rural household food security in Botswana. J. Hortic. For. 2011, 3, 171–177. [Google Scholar]
- Mariod, A.A.; Abdelwahab, S.I. Sclerocarya birrea (marula), an African tree of nutritional and medicinal uses: A review. Food Rev. Intern. 2012, 28, 375–388. [Google Scholar] [CrossRef]
- Magaia, T.; Uamusse, A.; Sjöholm, I.; Skog, K. Dietary fibre, organic acids, and minerals in selected wild edible fruits of Mozambique. Springerplus 2013, 2, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magaia, T.; Uamusse, A.; Sjöholm, I.; Skog, K. Proximate analysis of five wild fruits of Mozambique. Sci. World J. 2013, 2013, 601435. [Google Scholar] [CrossRef] [Green Version]
- Ondiek, J.O.; Abdulrazak, S.A.; Njoka, E.N. Chemical and mineral composition, in-vitro gas production, in-sacco degradation of selected indigenous Kenyan browses. Livest. Res. Rural Dev. 2010, 22, 2010. [Google Scholar]
- Maroyi, A. Medicinal uses, biological and chemical properties of Wild Plum (Harpephyllum caffrum): An indigenous fruit plant of Southern Africa. J. Pharm. Nutr. Sci. 2019, 9, 258–268. [Google Scholar]
- Wilson, A.L.; Downs, C.T. Fruit nutritional composition and non-nutritive traits of indigenous South African tree species. S. Afr. J. Bot. 2012, 78, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Wehmeyer, A.S. Nutrient composition of some edible wild fruits found in the Transvaal. S. Afr. Med. J. 1966, 40, 1102–1104. [Google Scholar] [PubMed]
- Malaisse, F.; Parent, G. Edible wild vegetable products in the Zambezian woodland area: A nutritional and ecological approach. Ecol. Food Nutr. 1985, 18, 43–82. [Google Scholar] [CrossRef]
- Ngemakwe, P.H.N.; Remize, F.; Thaoge, M.L.; Sivakumar, D. Phytochemical and nutritional properties of underutilised fruits in the Southern African region. S. Afr. J. Bot. 2017, 113, 137–149. [Google Scholar] [CrossRef]
- Stadlmayr, B.; Charrondière, U.R.; Eisenwagen, S.; Jamnadass, R.; Kehlenbeck, K. Nutrient composition of selected indigenous fruits from sub-Saharan Africa. J. Sci. Food Agric. 2013, 93, 2627–2636. [Google Scholar] [CrossRef]
- Yisa, J.; Egila, J.N.; Darlinton, A.O. Chemical composition of Annona senegalensis from Nupe land, Nigeria. Afr. J. Biotech. 2010, 9, 4106–4109. [Google Scholar]
- Moodley, R.; Koorbanally, N.; Jonnalagadda, S.B. Elemental composition and fatty acid profile of the edible fruits of Amatungula (Carissa macrocarpa) and impact of soil quality on chemical characteristics. Anal. Chim. Acta 2012, 730, 33–41. [Google Scholar] [CrossRef]
- Ohiokpehai, O. Promoting the nutritional goodness of traditional food products. Pak. J. Nutr. 2003, 2, 267–270. [Google Scholar]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Segu, K. Phoenix reclinata Jacq; Record from, PROTA4U; Brink, M., Achigan-Dako, E.G., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources Végétales de L’afrique Tropicale): Wageningen, The Netherlands, 2011. [Google Scholar]
- Benhura, C.; Benhura, M.A.N.; Muchuweti, M.; Nyagura, S.F.; Gombiro, P.E. Proximate analysis of Parinari curatellifolia fruit pulp of fruit from parts of Harare and a rural area in Zimbabwe. Pak. J. Nutr. 2012, 11, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Ogungbenle, H.N.; Atere, A.A. The chemical, fatty acid and sensory evaluation of Parinari curatellifolia seeds. Br. Biotech. J. 2014, 4, 379–386. [Google Scholar] [CrossRef]
- Joseph, K.S.; Bolla, S.; Joshi, K.; Bhat, M.; Naik, K.; Patil, S.; Bendre, S.; Gangappa, B.; Haibatti, V.; Payamalle, S.; et al. Determination of chemical composition and nutritive value with fatty acid compositions of African mangosteen (Garcinia livingstonei). Erwerbs Obstbau 2017, 59, 195–202. [Google Scholar] [CrossRef]
- Van Damme, P.; Van Den Eynden, V. Succulent and xerophytic plants used by the Topnaar of Namibia. Haseltonia 2000, 7, 53–62. [Google Scholar]
- Anhwange, B.A.; Ikyenge, B.A.; Nyiatagher, D.T.; Ageh, J.T. Chemical analysis of Citrullus lanatus (Thunb.) Matsum. &Nakai, Cucumeropsis mannii Naudin and Telfairia occidentalis Hook F. seeds oils. J. Appl. Sci. Res. 2010, 3, 265–268. [Google Scholar]
- Fila, W.A.; Itam, E.H.; Johnson, J.T.; Odey, M.O.; Effiong, E.E.; Dasofunjo, K.; Ambo, E.E. Comparative proximate compositions of watermelon Citrullus lanatus, squash Cucurbita pepo and rambutan Nephelium lappaceum. Intern. J. Sci. Technol. 2013, 2, 81–88. [Google Scholar]
- Velimpini, K.; Perkins, J.S. Integrating indigenous technical knowledge and modern scientific knowledge for biodiversity conservation and human livelihoods in the southern Kalahari. Botsw. Notes Rec. 2008, 39, 75–88. [Google Scholar]
- Bosch, C.H. Coccinia sessilifolia (Sond.) Cogn.; Record from, PROTA4U; Grubben, G.J.H., Denton, O.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources Végétales de L’afrique Tropicale): Wageningen, The Netherlands, 2004; Available online: https://www.prota4u.org/database/protav8.asp?fr=1&g=pe&p=Coccinia+sessilifolia+(Sond.)+Cogn (accessed on 19 March 2021).
- Wilkins-Ellert, M.H. Cucumis metuliferus E.Mey. ex Naudin; Record from, PROTA4U; Grubben, G.J.H., Denton, O.A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources Végétales de L’afrique Tropicale): Wageningen, The Netherlands, 2004; Available online: https://prota4u.org/database/protav8.asp?g=pe&p=Cucumis+metuliferus+E.Mey.+ex+Naudin (accessed on 19 March 2021).
- Ezeagu, I.E.; Metges, C.C.; Proll, J.; Petzke, K.J.; Akinsoyinu, A.O. Chemical composition and nutritive value of some wild-gathered tropical plant seeds. Food Nutr. Bull. 1996, 17, 1–4. [Google Scholar] [CrossRef]
- Petzke, K.J.; Ezeagu, I.E.; Proll, J.; Akinsoyinu, A.O.; Metges, C.C. Amino acid composition, available lysine content and in vitro protein digestibility of selected tropical crop seeds. Plant Foods Hum. Nutr. 1997, 50, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Chaka, B.; Osano, A.O. Analysis of selected nutrient levels at different growth stages of Dovyalis caffra (Kei-apple) fruits. Intern. J. Res. Innov. Appl. Sci. 2019, 4, 10–18. [Google Scholar]
- Ngadze, R.T.; Linnemann, A.R.; Nyanga, L.K.; Fogliano, V.; Ruud Verkerk, R. Local processing and nutritional composition of indigenous fruits: The case of monkey orange (Strychnos spp.) from Southern Africa. Food Rev. Intern. 2017, 33, 123–142. [Google Scholar] [CrossRef] [Green Version]
- Mojeremane, W.; Tshwenyane, S.O. Azanza garckeana: A valuable edible indigenous fruit tree of Botswana. Pak. J. Nutr. 2004, 3, 264–267. [Google Scholar]
- Nkafamiya, I.I.; Ardo, B.P.; Osemeahon, S.A.; Akinterinwa, A. Evaluation of nutritional, non-nutritional, elemental content and amino acid profile of Azanza garckeana (Goron Tula). Br. J. Appl. Sci. Technol. 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Jacob, C.; Shehu, Z.; Danbature, W.L.; Karu, E. Proximate analysis of the fruit Azanza garckeana (“Goron Tula”). Bayero J. Pure Appl. Sci. 2016, 9, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Maroyi, A. Azanza garckeana fruit tree: Phytochemistry, pharmacology, nutritional and primary healthcare applications as herbal medicine. Rev. Res. J. Med. Plants 2017, 11, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Ogunlaja, O.O.; Moodley, R.; Baijnath, H.; Jonnalagadda, S.B. Elemental Distribution and Health Risk Assessment of the Edible Fruits of Two Ficus Species, Ficus sycomorus L. and Ficus burtt-davyi Hutch. Biol. Trace Elem. Res. 2020, 198, 303–314. [Google Scholar] [CrossRef]
- Maliehe, S.T. An Evaluation of Nutraceutical Components of Syzygium cordatum Fruits for the Treatment of Gastrointestinal tract Infections. Ph.D. Thesis, University of Zululand, Richards Bay, South Africa, 2015. [Google Scholar]
- Maroyi, A. Syzygium guineense (Willd.) DC. [Internet] Record from PROTA4U; Louppe, D., Oteng-Amoako, A.A., Brink, M., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2008. [Google Scholar]
- Feyssa, D.H.; Njoka, J.T.; Asfaw, Z.; Nyangito, M.M. Uses and management of Ximenia americana, Olacaceae in semi-arid East Shewa, Ethiopia. Pak. J. Bot. 2012, 44, 1177–1184. [Google Scholar]
- Eromosele, I.C.; Eromosele, C.O.; Akintoye, A.O.; Komolafe, T.O. Characterization of oils and chemical analyses of the seeds of wild plants. Plant Foods Hum. Nutr. 1994, 46, 361–365. [Google Scholar] [CrossRef]
- Goosen, J.; Oosthuizen, D.; Stander, M.A.; Dabai, A.I.; Pedavoah, M.-M.; Usman, G.O. Phenolics, organic acids and minerals in the fruit juice of the indigenous African sourplum (Ximenia caffra, Olacaceae). S. Afr. J. Bot. 2018, 119, 11–16. [Google Scholar] [CrossRef]
- Feyssa, D.H.; Njoka, J.T.; Asfaw, Z.; Nyangito, M.M. Nutritional value of Berchemia discolor: A potential to food nutrition security of households. J. Biol. Sci. 2012, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.P.; Maina, H.A.; Friday, O.O. Determination of the nutritive values of Ziziphus mucronata obtained from Bagale Hills of Girei Local Government area of Adamawa State, Nigeria. J. Appl. Chem. 2018, 9, 105–108. [Google Scholar] [CrossRef]
- Maroyi, A. Nutraceutical and ethnopharmacological properties of Vangueria infausta subsp. infausta. Molecules 2018, 23, 1089. [Google Scholar] [CrossRef] [Green Version]
- Mothapo, M.J.; Mafeo, T.P.; Mamphiswana, N.D. Physico-chemical properties and selected nutritional components of wild medlar (Vangueria infausta) fruit harvested at two harvesting times. World J. Dairy Food Sci. 2014, 9, 79–85. [Google Scholar]
- Karau, G.M.; Njagi, E.N.; Machocho, A.K.; Wangai, L.N. Phytonutrient, mineral composition and in vitro antioxidant activity of leaf and stem bark powders of Pappea capensis (L.). Pak. J. Nutr. 2012, 11, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Microsoft JET Database Engine. Available online: https://www.prota4u.org/database/protav8.asp?g=psk&p=Englerophytum+magalismontanum+(Sond.)+T.D.Penn (accessed on 6 March 2021).
- Chivandi, E.; Davidson, B.; Pretorius, B.; Erlwanger, K. Proximate, mineral, amino acid, fatty acid, vitamin E, phytate phosphate and fibre composition of Mimusops zeyheri (Red Milkwood) seed. Intern. J. Food Sci. Technol. 2011, 46, 555–560. [Google Scholar] [CrossRef]
- Codron, D.; Lee-Thorp, J.A.; Sponheimer, M.; Codron, J. Nutritional content of savannah plant foods: Implications for browser/grazer models of ungulate diversification. Eur. J. Wildl. Res. 2007, 53, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Malebana, I.M.M.; Nkosi, B.D.; Erlwangera, K.H.; Chivandia, E. A comparison of the proximate, fibre, mineral content, amino acid and the fatty acid profile of Marula (Sclerocarya birrea caffra) nut and soyabean (Glycine max) meals. J. Sci. Food Agric. 2018, 98, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, 4490. [Google Scholar] [CrossRef] [Green Version]
- Ganong, W.F. Review of Medical Physiology; McGraw-Hill: New York, NY, USA, 2003; Volume 3, p. 17. [Google Scholar]
- AOAC. Official Methods of Analysis Association of Official Analytical, Chemists, 15th ed.; AOAC International: Washington DC, USA, 1990. [Google Scholar]
- Hoffman, J.R.; Falvo, M.J. Protein—Which is Best? J. Sport. Sci. Med. 2004, 3, 118–130. [Google Scholar]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; Report of an FAO Expert Consultation; FAO: Rome, Italy, 1989. [Google Scholar]
- Johnson, L.; Mander, A.P.; Jones, L.R.; Emmett, P.M.; Jebb, S.A. Energy-dense, low-fibre, high-fat dietary pattern is associated with increased fatness in childhood. Am. J. Clin. Nutr. 2008, 87, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Andrew, P.J.; Mayer, B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999, 43, 521–531. [Google Scholar] [CrossRef]
- Gokce, N. L-arginine and hypertension. J. Nutr. 2004, 134, 2807–2811. [Google Scholar] [CrossRef]
- Frezza, C.; Mauro, C. The metabolic challenges of immune cells in health and disease. Front. Immun. 2015, 6, 293. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Kessler, A.T.; Raja, A. Biochemistry, Histidine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538201/ (accessed on 6 March 2021).
- Manoli, I.; Venditti, C.P. Disorders of branched chain amino acid metabolism. Transl. Sci. Rare Dis. 2016, 1, 91–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef]
- Gu, C.; Mao, X.; Chen, D.; Yu, B.; Yang, Q. Isoleucine plays an important role for maintaining immune function. Curr. Protein Pept. Sci. 2019, 20, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Phenylalanine. In Food Science Technology. Nutrition Metabolism; Academic Press: Cambridge, MA, USA, 2003; pp. 314–321. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxtable, R.J. The Metabolism and functions of methionine. In Biochemistry of Sulfur. Biochemistry of the Elements; Springer: Boston, MA, USA; Volume 6, pp. 61–120. [CrossRef]
- Thrionine. Available online: https://aminoacidsguide.com/Thr.html (accessed on 13 March 2021).
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the Brain. J. Nutri. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists; Method 44–15A; AACC International: St. Paul, MN, USA, 2000; Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1115992 (accessed on 15 March 2021).
- AACC. Approved Methods of the American Association of Cereal Chemists; Method 08–01; AACC International: St. Paul, MN, USA, 1999; Available online: http://methods.aaccnet.org/summaries/08-10-01.aspx (accessed on 15 March 2021).
- AACC. Approved Methods of the American Association of Cereal Chemists; Method 71–10; AACC International: St. Paul, MN, USA, 1983; Available online: http://methods.aaccnet.org/summaries/30-25-01.aspx (accessed on 15 March 2021).
- Einarsson, S.; Josefsson, B.; Lagerkvist, S. Determination of amino acids with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1983, 282, 609–618. [Google Scholar] [CrossRef]
- FAO. Food Composition Table for Use in Africa; U.S. Department of Health, Education and Welfare, Nutrition Program and Food Consumption and Planning Branch; Food and Agriculture Organization of the United Nations: Rome, Italy, 1968. [Google Scholar]
- Asibey-Berko, E.; Tayie, F.A.K. Proximate analysis of some under-utilized Ghanian vegetables. Ghana J. Sci. 1999, 39, 91–96. [Google Scholar]
- USDA. National Food and Nutrient Analysis Program Wave; Nutrient Data Laboratory: Beltsville, MD, USA, 2018. [Google Scholar]


| Species | Common Name | Proximate (g/100 g) | Vitamin C (mg/100 g) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| P | F | Fat | A | C | M | ||||
| Anacardiaceae | |||||||||
| Harpephyllum caffrum Bernh. | Wild plum | 0.7 | 1.7 | 0.2 | 0.8 | 9.1 | 87.5 | - | [54,55,56] |
| Lannea edulis (Sond.) Engl. | Wild grape | 6.5 | 5.5 | 9.7 | 2.4 | 75.9 | - | 14 | [57] |
| Sclerocarya birrea (A.Rich.) Hochst. | Marula | 30.1 ± 0.2 | 10.5 ± 2.7 | 58.3 ± 1.3 | 3.8 ± 0.0 | 3.7 | 25.3% | 400 | [46,47,48,49,50,51,52,56,57,58,59] |
| Searsia undulata (Jacq.) T.S.Yi, A.J.Mill. & J.Wen. | Taaibos | - | - | - | - | - | - | - | - |
| Searsia discolor E. Mey. ex Sond. | - | - | - | - | - | - | - | - | - |
| Searsia pentheri Zahlbr. | - | - | - | - | - | - | - | - | - |
| Searsia dentata Thunb. | Nana berry | - | - | - | - | - | - | - | - |
| Annonaceae | |||||||||
| Annona senegalensis Pers. | Wild custard apple | 8.80% | 17.6% | 24% | 12.10% | 25.3% | 7.79% | - | [55,60] |
| Hexalobus monopetalus (A.Rich.) Engl. & Diels | Shakama plum | - | - | - | - | - | - | - | - |
| Apocynaceae | |||||||||
| Ancylobotrys capensis (Oliv.) Pichon | Wild apricot | 1.0 | 0.8 | 0.2 | 0.6 | 33.1 | 64.0 | - | [56,58] |
| Carissa macrocarpa (Eckl.) A.DC. | Natal plum | 0.74 ± 0.03 | - | 3.53 ± 0.01 | 0.50 ± 0.09 | 100.3 ± 0.2 | 78.45% | 168.36 | [61,62,63] |
| Sarcostemma viminale (L.) R.Br. | Caustic bush | - | - | - | - | - | - | - | - |
| Arecaceae | |||||||||
| Phoenix reclinata Jacq | Wild date palm | 0.2 | - | - | 0.4 | - | 22 + 109 | 6.5 | [64] |
| Asteraceae | |||||||||
| Chrysanthemoides monilifera (L.) Norl. | Bietou | - | - | - | - | - | - | - | - |
| Capparaceae | |||||||||
| Boscia albitrunca (Burch.) Gilg & Gilg-Ben. | Emigrant’s tea/Caper bush | - | - | - | - | - | - | - | - |
| Caryophyllaceae | |||||||||
| Pollichia campestris Aiton | Waxberry | - | - | - | - | - | - | - | - |
| Celastraceae | |||||||||
| Salacia kraussii (Harv.) Harv. | Ibontsi | 3.7 ± 0.00 | - | 1.5 ± 0.6 | 4.8 ± 0.2 | - | - | - | [54,57] |
| Chrysobalanceae | |||||||||
| Parinari curatellifolia Benth. | Mobola plum | 3.0% | 5.5% | 1.5% | 1.8% | 88.2% | - | 75 | [9,65,66] |
| Clusiaceae | |||||||||
| Garcinia livingstonei T.Anderson | African mangosteen | 0.65 ± 0.02% | 21.13 ± 0.02% | 2.56 ± 0.06% | 1.76 ± 0.04% | 95.02 ± 0.01% | 0.45 ± 0.13% | 7.2 | [67] |
| Cucurbitaceae | |||||||||
| Acanthosicyos horridus Welw. ex Hook.f. | Nara | 31% | - | - | - | - | - | - | [68] |
| Acanthosicyos naudiniana (Sond.) C.Jeffrey | Gemsbok cucumber | - | - | - | - | - | - | - | - |
| Citrullus lanatus (Thunb.) Matsum. & Nakai | Tsamma | 0.44 ± 0.05 | 0.19 ± 0.01 | 0.15 ± 0.01 | 0.21 ± 0.01 | 7.19 ± 0.05 | 91.92 ± 0.01 | - | [69,70,71] |
| Coccinia sessilifolia (Sond.) Cogn | Red gherkin | 2.1 | 1.3 | 0.2 | 0.2 | 13 | 82.3 | 25 | [72] |
| Cucumis anguria L. | Gherkin | - | - | - | - | - | - | - | - |
| Cucumis metuliferus E.Mey. ex Naudin | Jelly melon | 1.8 | 1.1 | 1.3 | - | 8 | 88.97 | 5.3 | [73] |
| Cucumis myriocarpus Naud | - | - | - | - | - | - | - | - | |
| Ebenaceae | |||||||||
| Diospyros mespiliformis Hochst. ex A.DC. | Jackal berry | 10.26 ± 0.01% | 2.00 ± 0.13% | 3.51 ± 0.18% | 3.00 ± 0.20% | 75.23 ± 0.40% | 6.01 ± 0.00% | - | [74,75] |
| Euclea crispa (Thunb.) Gϋrke | Bush guarri | - | - | - | - | - | - | - | - |
| Euphorbiaceae | |||||||||
| Bridelia mollis Hutch. | Velvet sweet berry | - | - | - | - | - | - | - | - |
| Uapaca kirkiana Müll.Arg. | Sugarplum | 1.8% | 8.4% | 1.1% | 2.2% | 86.5% | - | - | [9,59] |
| Fabaceae | |||||||||
| Cordyla africana Lour. | Wild mango | - | - | - | - | - | - | 75.6 | [58] |
| Flacourtiaceae | |||||||||
| Dovyalis caffra (Hook.f. & Harv.) Sim | Kei-apple | 0.4 | 0.7 | 0.4 | 0.5 | 4.7 | 85.9 | 275.88 mg/g | [58,76] |
| Dovyalis longispina (Harv.) Warb. | Natal Kei-apple | - | - | - | - | - | - | - | - |
| Flacourtia indica (Burm.f.) Merr. | Governor’s plum | 4.2% | 5.7% | 3.6% | 5.7% | 80.7% | - | - | [9] |
| Hydnoraceae | |||||||||
| Hydrona africana Thunb. | Jakkalskos | - | - | - | - | - | - | - | - |
| Iridaceae | |||||||||
| Romulea rosea (L.) Eckl. | Frutangs | - | - | - | - | - | - | - | - |
| Lauraceae | |||||||||
| Cryptocarya wyliei Stapf | Red quince | - | - | - | - | - | - | - | - |
| Loganiaceae | |||||||||
| Strychnos spinosa Lam. | Green monkey apple | 5.4% | 17.6% | 31.2% | 42.1% | 28.7% | 78.8% | - | [9,56,58,60,76] |
| Malvaceae | |||||||||
| Azanza garckeana (F. Hoffm) Exell & Hillc. | Snot apple | 12.0% | 45.3% | 1.1% | 35.2% | 28.4% | 13.54% | 30.8 mg/100 g | [5,9,77,78,79,80,81] |
| Morella serrata Lam. | Lance-leaf wax berry | - | - | - | - | - | - | - | - |
| Mesembryanthemaceae | |||||||||
| Carpobrotus edulis (L.) L.Bolus | Sour fig | - | - | - | - | - | - | - | - |
| Moraceae | |||||||||
| Ficus sycamorus L. | Sycamore fig | 5.6 ± 0.2% | 0.9% | 8.9 ± 0.5% | 4.4 ± 0.4% | 25.3 ± 1.1% | 55.8 ± 0.3% | - | [82] |
| Myrtaceae | |||||||||
| Syzygium cordatum Hochst.ex C.Krauss. | Water-berry | 5.91% | 1.7% | 7.7% | 2.3% | 63.9% | 23.8% | - | [42,56,83] |
| Syzygium guineense (Willd.) DC. | Water-berry | 10.1% | 30.3% | 4.0% | 7.1% | - | - | - | [84] |
| Olacaceae | |||||||||
| Ximenia americana L. | Blue sour plum | 7.26 ± 0.2% | 3.0 ± 0.01% | 13.0 ± 0.02% | 10.5 ± 0.1% | - | 64 ± 0.2% | 21.12 ± 0.01% | [57,85,86] |
| X. caffra Sond. | Sour plum | 3.1 | 0.7 | 1.3 | 1.4 | 26.3 | 49.2 | [9,87] | |
| Polygalaceae | |||||||||
| Nylandtia spinosa (L.) Dumort. | Skilpadbessie | - | - | - | - | - | - | - | - |
| Rhamnaceae | |||||||||
| Berchemia discolor (Klotzsch) Hemsl. | Brown ivory | 16.8% | 9.57% | 0.33% | 4.25% | 19.09% | 59.53% | 45.45% | [60,88] |
| Ziziphus mucronata Willd. | Buffalo-thorn | 35.10 ± 0.58% | 11.04 ± 0.88% | 27.40 ± 0.11% | 2.85 ± 0.23 | 23.61 ± 0.63% | 4.10 ± 0.32% | - | [46,89] |
| Rosaceae | |||||||||
| Rubus rigidus | Wild bramble | - | - | - | - | - | - | - | - |
| Rubiaceae | |||||||||
| Vangueria infausta Burch. | Wild medlar | 4.7 ± 0.4 | 26.3 ± 0.9 | 0.7 ± 0.2 | 5.7 ± 0.4 | 78.10% | 80.70% | 67.70% | [9,53,85,90,91] |
| Sapindaceae | |||||||||
| Pappea capensis Eckl. & Zeyh. | Jacket-plum | - | - | - | - | - | - | - | [35,36,92] |
| Sapotaceae | |||||||||
| Englerophytum magalismontanum (Sond.) T.D.Penn. | Stamvrug | - | - | - | - | 43 | - | 20 | [59,93] |
| Manilkara mochisia (Baker) Dubard | Lowveld milkberry | - | - | - | - | - | - | - | - |
| Mimusops zeyheri Sond. | Transvaal red milkwood | 9.3% | - | - | 4.1% | 2.0% | - | - | [94] |
| Scrophulariaceae | |||||||||
| Halleria lucida L. | White olive | - | - | - | - | - | - | - | - |
| Solanaceae | |||||||||
| Solanum retroflexum Dunal | Nightshade | - | - | - | - | - | - | - | - |
| Tiliaceae | |||||||||
| Grewia flava DC. | Velvet raisin bush | - | - | - | - | 82.1% | - | - | [62] |
| Verbenaceae | |||||||||
| Lantana rugosa Thunb. | Chameleon’s berry | - | - | - | - | - | - | - | - |
| Vitaceae | |||||||||
| Rhoicissus tomentosa (Lam.) Wild & R.B. Drumm. | Wild grape | 1.4 | - | - | - | - | - | - | [95] |
| Rhoicissus tridentata (L.f.) Wild & Drum. | Bushman’s grape | - | - | - | - | - | - | - | - |
| Taxon | Amino Acid (mg/100 g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histidine | Isoleucine | Leucine | Lysine | Methionine | Phenylanine | Threonine | Tyrosine | Valine | Ref. | |
| Annona senegalensis | - | 25 | 20 | 30 | 15 | 25 | 15 | 39 | 25 | [57] |
| Azanza garckeana | 3.67 | 4.98 | 12.01 | 11.78 | 2.00 | 8.00 | 4.78 | 4.89 | 6.00 | [81] |
| Diospyros mespiliformis | 2.3 | 3.7 | 5 | - | 1 | 3.3 | 3 | 2 | 4.3 | [75] |
| Mimusops zeyheri (seed) | 0.37 ± 0.06 | 0.38 ± 0.01 | 0.58 ± 0.01 | 0.64 ± 0.04 | 0.12 ± 0.01 | 0.34 ± 0.01 | 0.44 ± 0.00 | 0.71 ± 0.13 | 0.49 ± 0.00 | [94] |
| Sclerocarya birrea (nut) | 6.83 | 12.93 | 16.72 | 7.73 | 7.45 | 11.91 | 6.03 | 6.34 | 13.07 | [96] |
| Species | Proximate g/100 g | Energy kJ/100 g | |||||
|---|---|---|---|---|---|---|---|
| Ash | Fat | Fibre | Moisture | Protein | Carbohydrates | ||
| Carissa macrocarpa | 20.28 ± 0.12 l | 0.87 ± 0.05 h | 4.68 ± 0.22 f | 52.48 ± 0.00 e | 1.96 ± 0.00 f | 21.57 ± 0.01 l | 101.95 ± 0.00 k |
| Carpobrotus edulis | 8.76 ± 0.00 k | 0.21 ± 0.00 b | 11.46 ± 0.00 l | 19.61 ± 0.03 a | 3.45 ± 0.00 j | 10.97 ± 0.00 e | 59.57 ± 0.00 e |
| Cordyla africana | 1.97 ± 0.00 a | 3.55 ± 0.00 l | 3.06 ± 0.00 c | 71.48 ± 0.01 i | 0.004 ± 0.02 a | 19.94 ± 0.00 j | 111.73 ± 0.00 l |
| Dovyalis caffra (EC) | 3.90 ± 0.00 f | 0.73 ± 0.00 g | 3.44 ± 0.00 d | 79.30 ± 0.00 k | 0.83 ± 0.00 b | 11.80 ± 0.00 h | 57.09 ± 0.00 d |
| Dovyalis caffra (NEL) | 2.50 ± 0.00 b | 0.003 ± 0.02 a | 2.48 ± 0.00 b | 79.26 ± 0.00 j | 1.56 ± 0.00 c | 14.20 ± 0.02 i | 64.27 ± 0.00 f |
| Dovyalis longispina | 6.73 ± 0.01 j | 1.49 ± 0.00 k | 1.88 ± 0.01 a | 70.51 ± 0.00 h | 1.94 ± 0.00 e | 30.91 ± 0.00 n | 144.81 ± 0.00 m |
| Englerophytum magalismontanum | 2.05 ± 0.00 a | 0.31 ± 0.00 d | 5.60 ± 0.00 h | 88.11 ± 0.00 o | 0.83 ± 0.00 b | 3.10 ± 0.00 b | 18.51 ± 0.00 a |
| Garcinia livingstonei | 5.85 ± 0.00 i | 0.69 ± 0.01 f | 4.80 ± 0.00 g | 81.59 ± 0.00 l | 2.16 ± 0.00 g | 4.64 ± 0.00 c | 33.41 ± 0.00 c |
| Halleria lucida | 5.06 ± 0.00 h | 1.44 ± 0.00 j | 10.13 ± 0.01 k | 64.98 ± 0.00 g | 6.98 ± 0.00 m | 11.41 ± 0.00 f | 86.52 ± 0.01 i |
| Manilkara mochisia | 3.65 ± 0.00 d | 1.38 ± 0.00 i | 12.77 ± 0.00 m | 43.03 ± 0.00 b | 2.19 ± 0.00 h | 36.98 ± 0.00 o | 169.10 ± 0.00 o |
| Parinari curatellifolia | 3.69 ± 0.00 de | 0.25 ± 0.00 c | 8.88 ± 0.00 j | 63.39 ± 0.01 f | 2.61 ± 0.00 i | 21.18 ± 0.00 k | 97.37 ± 0.01 j |
| Pappea capensis | 3.42 ± 0.02 c | 5.11 ± 0.00 m | 16.51 ± 0.00 n | 45.63 ± 0.00 c | 4.33 ± 0.01 l | 25.00 ± 0.00 m | 163.31 ± 0.00 n |
| Phoenix reclinata | 4.23 ± 0.23 g | 1.44 ± 0.00 j | 29.89 ± 0.00 o | 48.92 ± 0.00 d | 4.08 ± 0.01 k | 11.44 ± 0.00 g | 75.04 ± 0.01 g |
| Syzygium cordatum | 3.79 ± 0.00 ef | 0.34 ± 0.00 e | 7.64 ± 0.00 i | 81.78 ± 0.00 m | 0.83 ± 0.00 b | 5.62 ± 0.03 d | 28.86 ± 0.02 b |
| Syzygium guineense | 3.34 ± 0.00 c | 7.74 ± 001 n | 3.84 ± 0.00 e | 82.41 ± 0.00 n | 1.66 ± 0.00 d | 1.01 ± 0.00 a | 80.34 ± 0.00 h |
| RDA (g/day) | |||||||
| FDA | 78 | 28 | 2.7–3.7 L | 50 | 275 | ±2000 kcal/day | |
| WHO | ≤5.0 mg/100 g | 44–77 | 18–35 | 2.1–2.6 L | 28–65 | 130 | 1900–2900 kcal/day |
| Amino Acid | RDA (FDA) | RDA (WHO) | Source of Amino Acid (g/100 g) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cm | Ce | Ca | Dc (EC) | Dc (NEL) | Dl | Em | Gl | Hl | Mm | Pc | Pcu | Pr | Sc | Sg | |||
| Arginine | N/A | N/A | 0.35 | 0.30 | 0.29 | 0.39 | 0.50 | 3.31 | 0.39 | 0.47 | 0.55 | 0.30 | 0.51 | 0.25 | 0.67 | 0.52 | 0.38 |
| Serine | N/A | N/A | 0.20 | 0.22 | 0.28 | 0.24 | 0.33 | 1.14 | 0.32 | 0.32 | 0.64 | 0.21 | 0.46 | 0.44 | 0.31 | 0.23 | 0.36 |
| Aspartic acid | N/A | N/A | 0.43 | 3.34 | 0.47 | 0.45 | 0.66 | 0.52 | 0.80 | 0.62 | 0.62 | 0.34 | 0.75 | 0.51 | 0.81 | 0.55 | 0.55 |
| Glutamic acid | N/A | N/A | 0.48 | 0.51 | 0.64 | 0.57 | 0.75 | 0.62 | 0.71 | 0.74 | 0.85 | 0.45 | 1.10 | 1.63 | 1.07 | 0.58 | 0.62 |
| Glycine | N/A | N/A | 0.17 | 0.37 | 0.22 | 0.19 | 0.26 | 0.24 | 0.24 | 0.31 | 0.46 | 0.20 | 0.38 | 0.27 | 0.31 | 0.22 | 0.29 |
| Threonine | 20 | 15 | 0.17 | 0.16 | 0.22 | 0.20 | 0.28 | 0.23 | 0.21 | 0.26 | 0.30 | 0.17 | 0.31 | 0.25 | 0.27 | 0.19 | 0.23 |
| Alanine | N/A | N/A | 0.21 | 0.16 | 0.34 | 0.28 | 0.40 | 0.29 | 0.29 | 0.36 | 0.40 | 0.23 | 0.41 | 0.65 | 0.36 | 0.31 | 0.34 |
| Tyrosine | 33 * | 25 * | 0.05 | 0.10 | 0.14 | 0.04 | 0.04 | 0.16 | 0.38 | 0.47 | 0.27 | 0.11 | 0.22 | 0.18 | 0.19 | 0.14 | 0.19 |
| Proline | N/A | N/A | 0.15 | 0.18 | 0.24 | 0.19 | 0.28 | 0.25 | 0.26 | 0.28 | 0.27 | 0.19 | 0.34 | 0.24 | 0.23 | 0.19 | 0.21 |
| HO-Proline | N/A | N/A | 0.40 | 0.14 | 0.19 | 0.07 | 0.08 | 0.09 | 0.16 | 0.06 | 0.07 | 0.06 | 0.14 | 0.17 | 0.06 | 0.11 | 0.08 |
| Methionine | 33^ | 15^ | 0.07 | 0.05 | 0.05 | 0.04 | 0.08 | 0.10 | 0.09 | 0.08 | 0.08 | 0.05 | 0.15 | 0.05 | 0.11 | 0.06 | 0.07 |
| Valine | 24 | 39 | 0.17 | 0.14 | 0.29 | 0.22 | 0.31 | 0.27 | 0.31 | 0.37 | 0.39 | 0.23 | 0.37 | 0.32 | 0.37 | 0.25 | 0.30 |
| Phenylalanine | 33 * | 25 * | 0.14 | 0.12 | 0.21 | 0.18 | 0.24 | 0.22 | 0.22 | 0.28 | 0.31 | 0.15 | 0.30 | 0.22 | 0.27 | 0.20 | 0.24 |
| Isoleucine | 19 | 20 | 0.14 | 0.12 | 0.23 | 0.16 | 0.22 | 0.21 | 0.23 | 0.27 | 0.30 | 0.17 | 0.29 | 0.23 | 0.24 | 0.19 | 0.23 |
| Leucine | 42 | 39 | 0.23 | 0.18 | 0.31 | 0.27 | 0.36 | 0.36 | 0.35 | 0.46 | 0.47 | 0.28 | 0.48 | 0.34 | 0.42 | 0.32 | 0.39 |
| Histidine | 14 | 10 | 0.14 | 0.18 | 0.26 | 0.08 | 0.08 | 0.16 | 0.24 | 0.28 | 1.56 | 0.23 | 0.63 | 0.27 | 0.31 | 0.12 | 0.62 |
| Lysine | 38 | 30 | 0.30 | 0.26 | 0.53 | 0.27 | 0.28 | 0.55 | 0.54 | 0.67 | 0.58 | 0.37 | 0.77 | 0.46 | 0.58 | 0.49 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibiya, N.P.; Kayitesi, E.; Moteetee, A.N. Proximate Analyses and Amino Acid Composition of Selected Wild Indigenous Fruits of Southern Africa. Plants 2021, 10, 721. https://doi.org/10.3390/plants10040721
Sibiya NP, Kayitesi E, Moteetee AN. Proximate Analyses and Amino Acid Composition of Selected Wild Indigenous Fruits of Southern Africa. Plants. 2021; 10(4):721. https://doi.org/10.3390/plants10040721
Chicago/Turabian StyleSibiya, Nozipho P., Eugenie Kayitesi, and Annah N. Moteetee. 2021. "Proximate Analyses and Amino Acid Composition of Selected Wild Indigenous Fruits of Southern Africa" Plants 10, no. 4: 721. https://doi.org/10.3390/plants10040721