A Cryptic Invader of the Genus Persicaria (Polygonaceae) in La Palma and Gran Canaria (Spain, Canary Islands)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Taxonomy
3.1.1. Based on Morphological Data
- Persicaria hydropiperoides (Michx.) Small, Fl. S.E. U.S. 378. 1903.
- ≡ Polygonum hydropiperoides Michx., Fl. Bor.-Amer. 1: 239. 1803.
- Protologue: Hab. in Pensylvana, Virginia, Carolina (U.S.A.). (Type: P).
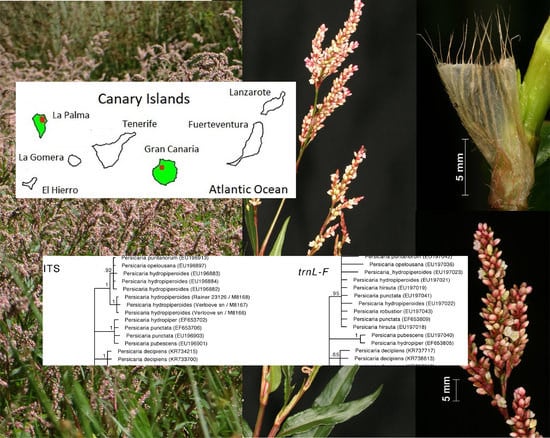
3.1.2. Phylogenetic Analyses
3.2. Primary and Secondary Distribution
3.3. Habitat and Ecology
3.4. Biostatus in the Canary Islands
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Specimens Examined
References
- Hedberg, O. Pollen morphology in the genus Polygonum LSI and its taxonomical significance. Sven. Bot. Tidskr. 1946, 40, 371–414. [Google Scholar]
- Haraldson, K. Anatomy and taxonomy in Polygonaceae subfamily Polygonoideae Meisn. emend. Jaretzky. Symb. Bot. Ups. 1978, 22, 1–95. [Google Scholar]
- Ronse Decraene, L.P.; Akeroyd, J.R. Generic limits in Polygonum and related genera (Polygonaceae) on the basis of floral characters. Bot. J. Linn. Soc. 1988, 98, 321–371. [Google Scholar] [CrossRef]
- Ronse Decraene, L.P.; Hong, S.P.; Smets, E. Systematic significance of fruit morphology and anatmy in tribes Persicarieae and Polygoneae (Polygonaceae). Bot. J. Linn. Soc. 2000, 134, 301–337. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.T.; Donoghue, M.J. Molecular phylogeny of Persicaria (Persicarieae, Polygonaceae). Syst. Bot. 2008, 33, 77–86. [Google Scholar] [CrossRef]
- Galasso, G.; Banfi, E.; Mattia, F.D.; Grassi, F.; Sgorbati, S.; Labra, M. Molecular phylogeny of Polygonum L. sl (Polygonoideae, Polygonaceae), focusing on European taxa: Preliminary results and systematic considerations based on rbcL plastidial sequence data. Atti Della Soc. Ital. Di Sci. Nat. E Del Mus. Civ. Di Stor. Nat. Di Milano 2009, 150, 113–148. [Google Scholar]
- Sanchez, A.; Schuster, T.M.; Kron, K.A. A large-scale phylogeny of Polygonaceae based on molecular data. Int. J. Plant Sci. 2009, 170, 1044–1055. [Google Scholar] [CrossRef]
- Burke, J.M.; Sanchez, A.; Kron, K.; Luckow, M. Placing the woody tropical genera of Polygonaceae: A hypothesis of character evolution and phylogeny. Am. J. Bot. 2010, 97, 1377–1390. [Google Scholar] [CrossRef]
- Sanchez, A.; Schuster, T.M.; Burke, J.M.; Kron, K.A. Taxonomy of Polygonoideae (Polygonaceae): A new tribal classification. Taxon 2011, 60, 151–160. [Google Scholar] [CrossRef]
- Schuster, T.M.; Reveal, J.L.; Kron, K.A. Phylogeny of Polygoneae (Polygonaceae: Polygonoideae). Taxon 2011, 60, 1653–1666. [Google Scholar] [CrossRef]
- Stanford, E.E. Possibilities of hybridism as a cause of variation in Polygonum. Rhodora 1925, 27, 81–89. [Google Scholar]
- Timson, J. A study of hybridization in Polygonum section Persicaria. J. Linn. Soc. 1964, 59, 155–160. [Google Scholar] [CrossRef]
- McDonald, C.B. A Biosystematic Study of the Polygonum hydropiperoides (Polygonaceae) Complex. Am. J. Bot. 1980, 67, 664–670. [Google Scholar] [CrossRef]
- Akeroyd, J.R. Two overlooked species of Polygonum from SE Europe. Bot. J. Linn. Soc. 1987, 95, 251–257. [Google Scholar] [CrossRef]
- Atha, D.E.; Nee, M.H.; Naczi, R.F.C. Persicaria extremiorientalis (Polygonaceae) is established in the flora of the eastern United States of America. J. Torrey Bot. Soc. 2010, 137, 333–338. [Google Scholar] [CrossRef]
- Mifsud, S. A study of the genus Persicaria Miller (Polygonaceae) in the Maltese Islands. Cent. Mediterr. Nat. 2012, 5, 26–51. [Google Scholar]
- Galasso, G.; Montoleone, E.; Federico, C. Persicaria senegalensis (Polygonaceae), entità nuova per la flora italiana, e chiave di identificazione delle specie del genere Persicaria in Italia. Nat. Hist. Sci. 2014, 1, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.T.; Donoghue, M.J.; Sultan, S.E. On the resurrection of Persicaria puritanorum (Polygonaceae). Phytotaxa 2017, 308, 20–36. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Chen, S.-F.; Chen, M.-L.; Li, B. Persicaria jucunda var. rotunda (Polygonaceae, Persicarieae), a distinct distylous taxa raised to specific rank. PhytoKeys 2019, 126, 127–138. [Google Scholar] [CrossRef]
- Acebes Ginovés, J.R.; León Arencibia, M.C.; Rodríguez Navarro, M.L.; del Arco Aguilar, M.; Gallo, A.G.; Pérez de Paz, P.L.; de la Torre, V.E.W. Pteridophyta, Spermatophyta. In Lista de Especies Silvestres de Canarias. Hongos, Plantas y Animales Terrestres; Arechavaleta, M., Rodríguez, S., Zurita, N., García, A., Eds.; Gobierno de Canarias: La Laguna, Spain, 2010; pp. 119–172. [Google Scholar]
- Lid, J. Contributions to the Flora of the Canary Islands; Universitetsforlaget: Olso, Norway, 1967; Volume 23, p. 212. [Google Scholar]
- Santos, A. Vegetacion y Flora de La Palma; Editorial Interinsular Canaria S.A.: Santa Cruz de Tenerife, Spain, 1983; p. 348. [Google Scholar]
- Morais, P.; Reichard, M. Cryptic invasions: A review. Sci. Total Environ. 2018, 613–614, 1438–1448. [Google Scholar] [CrossRef]
- Jarić, I.; Heger, T.; Monzon, F.C.; Jeschke, J.M.; Kowarik, I.; McConkey, K.R.; Pyšek, P.; Sagouis, A.; Essl, F. Crypticity in Biological Invasions. Trends Ecol. Evol. 2019, 34, 291–302. [Google Scholar] [CrossRef]
- Verloove, F. Invaders in disguise. Conservation risks derived from misidentification of invasive plants. Manag. Biol. Invasions 2010, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff—New York Botanical Garden’s Virtual Herbarium. Available online: http://sweetgum.nybg.org/ih/ (accessed on 4 January 2021).
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Kim, S.-T.; Donoghue, M.J. Incongruence between cpDNA and nrITS trees indicates extensive hybridization within Eupersicaria (Polygonaceae). Am. J. Bot. 2008, 95, 1122–1135. [Google Scholar] [CrossRef]
- Kartzinel, T.R.; Chen, P.A.; Coverdale, T.C.; Erickson, D.L.; Kress, W.J.; Kuzmina, M.L.; Rubenstein, D.I.; Wang, W.; Pringle, R.M. Metabarcoding illuminates dietary niche partitioning by African large herbivores. Proc. Natl. Acad. Sci. USA 2015, 112, 8019–8024. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Swofford, D.L. PAUP. Phylogenetic Analysis Using Parsimony (and Other Methods), version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002; p. 143. [Google Scholar]
- Johnson, L.A.; Soltis, D.E. Assessing congruence: Empirical examples from molecular data. In Molecular Systematics of Plants II: DNA Sequencing; Soltis, P.S., Soltis, D.E., Doyle, J.J., Eds.; Kluwer: Boston, MA, USA, 1998; pp. 297–348. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Huelsenbeck, J.; Ronquist, F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.J. Tracer v1.4. 2007. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 4 January 2021).
- Suzuki, Y.; Glazko, G.V.; Nei, M. Overcredibility of molecular phylogenies obtained by Bayesian phylogenetics. Proc. Nat. Acad. Sci. USA 2002, 99, 16138–16143. [Google Scholar] [CrossRef] [Green Version]
- Michaux, A. Flora Boreali-Americana; Levrault: Paris, France; Strasbourg, France, 1803; Volume 1, p. 330. [Google Scholar]
- Kunth, K.S. Polygonum. In Nova Genera et Species Plantarum, quarto ed.; Humboldt, F., Bonpland, A., Kunth, C.S., Eds.; Lutetiae Parisiorum: Paris, France, 1818; Volume 2, pp. 177–180. [Google Scholar]
- Stanford, E.E. Polygonum hydropiperoides and P. opelousanum. Rhodora 1926, 28, 11–17. [Google Scholar]
- Stanford, E.E. Polygonum hydropiperoides and P. opelousanum (continued from page 17). Rhodora 1926, 28, 22–29. [Google Scholar]
- Brandbyge, J. Polygonaceae. In Flora of Ecuador; Harling, G., Andersson, L., Eds.; Deptartment of Biological and Environmental Sciences, University of Gothenburg: Gothenburg, Sweden, 1989; Volume 38, p. 62. [Google Scholar]
- Standley, P.C.; Steyermark, J.A. Flora of Guatemala; Fieldiana, Botany; Chicago Natural History Museum: Chicago, IL, USA, 1946; Volume 24, p. 493. [Google Scholar]
- Cialdella, A.M. Estudio Taxonómico y Fitogeográfico de las Especies Argentinas del Género Polygonum L. (Polygonaceae). Ph.D. Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 1986. [Google Scholar]
- Cialdella, A.M. Revisión de las especies Argentinas de Polygonum s.l. (Polygonaceae). Darwiniana 1989, 29, 179–246. [Google Scholar]
- Kiesling, R.; Múlgara, M.E.; Ulibarri, E.A. (Eds.) Flora de San Juan; Vazquez Mazzini Editores: Buenos Aires, Argentina, 1994; Volume 1, p. 348. [Google Scholar]
- Thomas, J.R. Vegetative Key to Polygonum in Missouri. Missouriensis 2005, 26, 22–35. [Google Scholar]
- Small, J.K. A monograph of the North American species of the genus Polygonum. Mem. Dep. Bot. Columbia Coll. 1895, 1, 1–183. [Google Scholar]
- Scholz, H. Polygonaceae. In DICOT WEEDS 1 Dicotyledonous Weeds of 13 Families; Häfliger, T.J., Wolf, M., Eds.; Documenta Ciba Geigy: Basle, Switzerland, 1988; pp. 265–311. [Google Scholar]
- Hinds, H.R.; Freeman, C.C. Persicaria. In Flora of North America; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA, 2005; Volume 5, pp. 574–594. [Google Scholar]
- Graham, R.A. Polygonaceae. In Flora of Tropical East Africa; Turrill, W.B., Milne-Redhead, E., Eds.; Crown Agents for Overseas Governments & Administrations: London, UK, 1958; p. 11. [Google Scholar]
- Villar, L. Polygonum. In Flora iberica; Castroviejo, A., Lainz, M., López González, G., Montserrat, P., Muñoz Garmendia, F., Paiva, J., Villar, L., Eds.; Real Jardin Botánico, CSIC: Madrid, Spain, 1990; Volume 2, pp. 571–586. [Google Scholar]
- Stace, C. New Flora of the British Isles; Cambridge University Press: Cambridge, UK, 2010; p. 1232. [Google Scholar]
- Wilson, K.L. Some widespread species of Persicaria (Polygonaceae) and their allies. Kew Bull. 1990, 45, 621–636. [Google Scholar] [CrossRef]
- Hedberg, O. Polygonaceae. In Flora of Ethiopia and Eritrea; Edwards, S., Tadesse, M., Demissew, S., Hedberg, I., Eds.; Uppsala University: Addis Ababa, Ethiopia; Uppsala, Sweden, 2000; Volume 2(1), pp. lxiii–532. [Google Scholar]
- Holm, L.G.; Pancho, J.V.; Herberger, J.P.; Plucknett, D.L. A Geographical Atlas of World Weeds; John Wiley & Sons: New York, NY, USA, 1979; p. 391. [Google Scholar]
- Randall, R.P. A Global Compendium of Weeds, 3rd ed.; R.P. Randall: Perth, Australia, 2017; p. 3653. [Google Scholar]
- Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 2 January 2019).
- Trelease, W. Botanical observations on the Azores. Annu. Rep. Mo. Bot. Gard. 1897, 8, 77–220. [Google Scholar] [CrossRef]
- Hansen, A.; Sunding, P. Flora of Macaronesia. Check-list of vascular plants (4th revised edition). Sommerfeltia 1993, 17, 1–295. [Google Scholar] [CrossRef]
- Schäfer, H. Chorologie und Diversität der Flora der Azoren. Ph.D. Thesis, Universität Regensburg, Regensburg, Germany, 2013. [Google Scholar]
- Amaral Franco, J. A phytogeographical sketch of the Azores. Bol. Da Soc. Boteriana Ser. 2 1974, 47, 105–113. [Google Scholar]
- Schäfer, H. Flora of the Azores: A Field Guide; Margraf Publishers: Weikersheim, Germany, 2005; p. 346. [Google Scholar]
- Duke, J.A. Polygonaceae. In Flora of Panama; Part IV. Fascicle 3; Woodon, R.E., Jr., Schery, R.W., Eds.; Annals of the Missouri Botanical Garden 1960; Volume 47, pp. 305–359.
- Wiggins, I.L.; Porter, D.M. Flora of the Galapágos Islands; Stanford University Press: Stanford, CA, USA, 1971; p. 998. [Google Scholar]
- Kunkel, G. Sobre Plantas Vasculares de Gran Canaria. Cuad. Botánica Canar. 1969, 5, 5–12. [Google Scholar]
- Von Buch, L. Physicalische Beschreibung der Canarischen Inseln; Königlichen Akademie der Wissenschaften: Berlin, Germany, 1825; p. 434. [Google Scholar]
- Webb, P.B.; Berthelot, S. Histoire Naturelle des Îles Canaries; tome 3(2) sect. 1; Béthune: Paris, France, 1836. [Google Scholar]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [Green Version]







| P. hydropiperoides sensu [49] | P. persicarioides | |
|---|---|---|
| Racemes | Almost linear or narrowly cylindric, +/− interrupted, lax with single flowers +/− distinct and rachis partially visible. In this respect the inflorescence resembles the European species Persicaria hydropiper (L.) Delarbre | Narrowly oblong to cylindrical, +/− uninterrupted (except sometimes at base), moderately stout, loose to densely flowered with most flowers not distinct individually and rachis not or hardly visible. The inflorescence resembles the European species Persicaria maculosa in this respect |
| Ocrea bristles | 4–10 mm long | (2–)3–5(–7) mm long (reminiscent of P. maculosa) |
| Blade | Without dark blotch | With dark blotch (like P. maculosa) or sometimes without such a blotch |
| Stamens | 8 | 6–7(–8) (like P. maculosa) |
| Style | Homostylous, all styles three-parted | Heterostylous, styles two or three-parted (like P. maculosa) |
| Achenes | All trigonous, 1.5–3 mm long, included or apex exserted | Mostly lenticular and both sides flat or one side ± angular domed, more rarely trigonous (like P. maculosa), 2.1–2.4(–3) mm long, nearly always included |
| P. maculosa | P. hydropiperoides var. persicarioides | P. decipiens |
|---|---|---|
| Annual herb | Annual or perennial herb, seedlings are flowering and fruiting in the first year | Perennial herb |
| Racemes at maturity (5–)10–30(–60) × 7–12 mm, erect, cylindrical and uninterrupted (except sometimes at the very base), obtuse, stout, flowers densely crowded and concealing each other, therefore rachis and each flower not individually distinct | Racemes at maturity (10–)20–40(–60) × 5–8 (–10) mm, erect to pendent, narrowly oblong to cylindrical and ± uninterrupted (except sometimes at base), ± obtuse, moderately stout, loose to densely flowered with most flowers not distinct individually and rachis not or hardly visible | Racemes at maturity 20–60(–90) mm × 6–8 mm, erect, narrowly cylindrical, obtuse, lax and slender, ± interrupted at base, with each flower and the rachis at least partially visible |
| Stem green to brownish, sometimes reddish, to 1 m, erect, ascending or decumbent, simple or mostly ± squarrosely branched, sometimes rooting at nodes | Stem green, often completely tinged with purple red or crimson throughout, to 1 (–2,5) m, erect or decumbent at the base to ascending, often rooting at the nodes and creeping, simple or branched above the base or richly so from the base | Stem green, becoming brown below, to 1 m, erect or basally decumbent, rooting at the lower nodes, simple or sparsely branched, lateral branches strongly upright |
| Rhizomes and stolons absent, root spindle-like | Rhizomes usually present, often thick, long creeping and generating colonies; weaker rhizomes and epigeous creeping stems produced in moist to wet substrate | Rhizomes present, sometimes also stolons |
| Leaf base tapered or cuneate to acute, blade ovate-lanceolate to lanceolate, black blotch adaxially usually present | Leaf base tapered or cuneate to acute, blade broadly-lanceolate to linear-lanceolate, black blotch adaxially present or absent | Leaf base contracted and ± rounded, blade narrowly lanceolate-elliptic, ± long acuminate, black blotch adaxially absent |
| Ocrea glabrous or strigose, often with small, thin, spreading hairs; bristles (0.2–) 1.3–2(–3.5) mm long | Ocrea with (0.5–)1(–1.5) mm long, ± distinctly broad-based, coarse, strigose hairs of variable density, rarely glabrous; bristles (2–)3–5(–7) mm long | Ocrea glabrous or ± thinly covered with closely ascending, bristly hairs 1–1.25 mm long; bristles (3–)5–12(–25) mm long |
| Achenes (2–)2.5–2.8(–3.2) mm, mostly lenticular and both sides flat or one side ± angular domed, more rarely trigonous, mixed in the same raceme | Achenes (2–)2.1–2.4(–3) mm, rarely over 2,5 mm long, mostly lenticular and both sides flat or one side ± angular domed, more rarely trigonous, mixed in the same raceme but the trigonous ones always much less in number | Achenes (2–)2.5–3 mm, trigonous (rarely obscurely lenticular). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verloove, F.; Otto, R.; Janssens, S.; Kim, S.-T. A Cryptic Invader of the Genus Persicaria (Polygonaceae) in La Palma and Gran Canaria (Spain, Canary Islands). Diversity 2021, 13, 551. https://doi.org/10.3390/d13110551
Verloove F, Otto R, Janssens S, Kim S-T. A Cryptic Invader of the Genus Persicaria (Polygonaceae) in La Palma and Gran Canaria (Spain, Canary Islands). Diversity. 2021; 13(11):551. https://doi.org/10.3390/d13110551
Chicago/Turabian StyleVerloove, Filip, Rainer Otto, Steven Janssens, and Sang-Tae Kim. 2021. "A Cryptic Invader of the Genus Persicaria (Polygonaceae) in La Palma and Gran Canaria (Spain, Canary Islands)" Diversity 13, no. 11: 551. https://doi.org/10.3390/d13110551