Manuscript accepted on : 07-Oct-2021
Published online on: 13-10-2021
Plagiarism Check: Yes
Reviewed by: Dr. Hager Tarek
Second Review by: Dr. Aisha Siddiqi
Final Approval by: Dr. Hifzur R. Siddiqui
Effect of Extract of Uria Picta Jacq. on Experimentally Induced Hypertension in Wistar Rats
Pavan Bhausaheb Udvant1 , Shubham Jagdish Khairnar2*
, Shubham Jagdish Khairnar2* , Ghansham Balakrishn Jadhav1
, Ghansham Balakrishn Jadhav1 , Rahul Ramakant Sable1
, Rahul Ramakant Sable1 and Mourya Krishnakumar Arprit1
and Mourya Krishnakumar Arprit1
1Department of Pharmacology, MVP’s College of Pharmacy, MVP campus, Gangapur Road, Nashik, Affiliated to S.P. Pune University, (M.S.), India.
2Department of Pharmacology, MET’S Institute of Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, Nashik.
Corresponding Author E-mail: shubhamkiop@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2937
ABSTRACT: Many allopathic medicines demand to remedy for hypertension but fail to fulfill the purpose, because of side effects and high cost. Herbal medicine does not cause side effects and natural herbs are completely safe. Rational and purpose of this study was to see how effective Uraria picta. Jacq. (U.P) extract was on experimentally induced hypertension in rats. The purpose of this research was to explore if U. Picta could be utilized as a curative or preventative medicine, as well as to investigate U. Picta possible toxicity in these animals. Blood pressure was elevated by Angiotensin II (150 ug/kg, i.p) in rats that is hypertensive condition. Animals were divided into groups as follow Group I (control) - vehicle, Group II Angiotensin II (150µgkg), Group III Angiotensin II extract low dose (100mgkg), Group IV-Angiotensin II extract middle dose (200mgkg), Group V-Angiotensin II extract (400mg/kg), Group VI – (Angiotensin II Telmisartan0.8mg/kg). and dosed as per protocol. Blood pressure was monitored with the noninvasive techniques (NIBP) using Power lab (AD instrument) Australia. Effect of extracts was studied on various oxidative stress markers like SOD, CAT, LOP and NO. as per observation extract of U. picta of high dose (400mg/kg BW p.o) had shown significant alteration in endogenous antioxidant entities i.e., SOD, CAT, LPO and also reduced NO level indicate antioxidant property of U. Picta. Same of above dose also reduced blood pressure and ACE level. So, from the above outcome we can consider that U.Picta extract may be having antioxidant and antihypertensive activity.
KEYWORDS: Angiotensin-II; ACE; Catalase; Hypertension; Oxidative Stress; Uraria picta. jacq
Download this article as:| Copy the following to cite this article: Udvant P. B, Khairnar S. J, Jadhav G. B, Sable R. R, Arprit M. K. Effect of Extract of Uria Picta Jacq. on Experimentally Induced Hypertension in Wistar Rats. Biosci Biotech Res Asia 2021;18(3). |
| Copy the following to cite this URL: Udvant P. B, Khairnar S. J, Jadhav G. B, Sable R. R, Arprit M. K. Effect of Extract of Uria Picta Jacq. on Experimentally Induced Hypertension in Wistar Rats. Biosci Biotech Res Asia 2021;18(3). Available from: https://bit.ly/3iXxXR2 |
Introduction
Angina pectoris, myocardial infarction, stroke, renal failure, and heart failure are all symptoms of systemic arterial hypertension, it is one of the most well-known ischemic heart disease risk factors and manifestations. 1 High bp is seen as a property rather than a sickness, and it deviates from the norm quantitatively rather than qualitatively. Systemic BP rises due hypervolemic condition (systemic fluid retention) and age within incidences of cardiopathy (particularly stroke, ischemic heart diseases such as myocardial infarction, angina Pectoris and atherosclerosis) is closely related to mean normal BP range at all type of patients (pediatric or geriatric), even when BP readings are within the so-called ‘normal range’. 2 Hypertension is very relatable with vascularity and their physiology associated with an endothelial dysfunction and oxidative stress. 3 According to the pooled analysis of available regional data and national data the incidences of hypertension are 1billion in 2000, and this proportion may be increases up to 29% by 2025. It also shows that both genders are equally susceptible to hypertensive condition, and that eventually spread worldwide. 4 Worldwide prevalence estimates for hypertension may be near about 1 billion individual population, and approximately 7.1 million deaths yearly may be attributable to hypertension and 4.5% of the disease burden [64 million disability adjusted life years (DALYs)]. Hypertension accounts for a considerable share of the worldwide illness burden.5 In1990, 467 percent of deaths from cardiovascular disorders occurred before the age of 70 in poor nations, compared to 265 percent in industrialized countries.21
 |
Figure 1: Uraria Picta Jacq. |
Taxonomical classification:
Kingdom: Plantae
Phylum: Tracheophyta
Class: Magnoliopsida
Order: Fabales
Family: Leguminosae
Genus: Uraria
Species: Uraria picta Jacq.
Uraria picta Jacq. is a medicinal plant belongs to family Leguminosae. It is an erect annual woody herb. Some people have classified it as perennial undershrub. In the past, the plant was utilized for a number of uses. It has been reported with number of qualities and pharmacological activities such as anti-inflammatory 22,23,24, hepatoprotective 25, antimicrobial 26, antidiarrheal 27, antioxidant 28, anticholinesterase 29,30, anxiolytic and psychomimetic activity 31,32,33. Decoction of the Plant has many benefits incough, chill, cold, and fever cases down good results in treatment of gonorrhea. The plant is also useful as antidiuretic 34,35.
Materials and Methods
Drug and chemicals
The plant extract of U.pictareceived and authenticated from VHCA Ayurveda LLP (office near bus stand, G.T road Gharunda- 132114, Haryana).
Experimental requirement
The chemicals and resource kit used were analytical grade and purchased from well-known suppliers. Angiotensin II purchased from Sigma Aldrich laboratory, Mumbai; Telmisartan purchased from Fidelity Life sciences Pvt.Ltd, India; biochemical kits procured from Auto span, surat, India; and U. picta purchased from VHCA Ayurveda LLP.
The primary phytochemical analysis was performed by using methods of Dr. Kokate C. K 7.
Phenolic and Tannins compound test
The presence of tannins and phenolic chemical entities was determined by mixing 2-3ml of test solution with 5% ferric chloride solution. A deep black blue color indicated the presence of tannins and phenolic chemical entities. 37
Total phenol and Flavonoid assay
Total flavonoid content 8
The Flavonoids content was estimated by aid of aluminium chloride with the help of colorimetry. Rutin was used to create a standard calibration curve. 10 mg of rutin was solubilized in 80% ethanol, and then various dilutions were prepared according to protocol. To make up the volume, the diluted standard solutions (0.5 ml) were individually mixed with 95 percent C2H6OH, 10% AlCl3, 1 molar potassium acetate, and distilled water. The same concentrations of Uraria picta. jacq extract were also prepared. After half an hour of incubation at room temperature, the absorbance of the reaction mixture was measured with a spectrophotometer at 415 nm. The total flavonoid content of the test extract was calculated using a standard calibration curve calculation. 37
Total phenolic content 9
Folin’s phenol reagent (F C) was used to quantify the total soluble phenolic compounds present in U.Picta extract. Gallic acid (GA) solutions at varied concentrations of0,50,100,150,250, and 500 g/ml in ethanol were used to create the calibration curve. 1 mL Folin’s phenol reagent was added to 0.1 mL U. Picta extract (1 mg/mL in distilled water). After vigorous shaking for 2 hours at room temperature, 3 mL of 2 percent sodium bicarbonate was added, and the absorbance was measured using a spectrophotometer at 760 nm. All tests were performed in 3 times and mean were taken in consideration. Gallic acid equivalent (mg/g dry mass) was used to express total phenolic content. 37
Animals
Male Wistar rats weighing 200-230g were purchased from Laboratory Animal Centre for Scientific and Medical Innovation (LACSMI) BIOFARMS PVT. LTD. Alephata, Pune, India for the experimental purpose. They were kept in Polypropylene cages with husk bedding, which was clean and replace every week. Animals were housed in 12hour light and 12hour dark cycle condition. Temperature was maintained at about 25±5℃. The animals were fed commercial pellets and had unlimited access to water. The experiment followed the criteria of the Committee for the Purpose of Control and Supervision of Animals (CPCSEA) of New Delhi, India, and the Institutional Animal Ethical Committee (IAEC) of this study (IAEC/2019/04) accepted the protocol.
Acute toxicity studies 10
The OECD (Organization for Economic Co-operation and Development) guidelines No. 420 were used to determine the acute toxicity test of rats and mice were put into five groups (n=6) after fasting overnight. The doses were given to the animals in the following order:50,100,200,00,00,1000,1500, and 2000 mg/kg body weight. After administration of doses of U.picta signs of toxicity and mortality rate were observed every hour for the first 6h and every day for two weeks.
Induction of hypertension:
Rats were administered with Angiotensin II (150 ug/kg, i.p) 2-6 weeks. To induce hypertension. The average weight animal (200-230g) was included in study.
Experimental design
The animals were assigned to six groups (n=6).
Group I (control) – vehicle,
Group II Angiotensin II (150 µg/kg BW p.o),
Group III Angiotensin II + extract low dose (100mg/kg BW p.o),
Group IV-Angiotensin II + extract middle dose (200mg/kg BW p.o),
Group V-Angiotensin II + extract High dose (400mg/kg BW p.o),
Group VI – (Angiotensin II +Telmisartan 0.8mg/kg BW p.o).
After inducing hypertension, the medications (test and Standard) were dosed on a regular basis for four weeks.11, 12, 13
In vivo experimental method
After induction of hypertension, the treatments were initiated. each animal was weighed before start of treatment and thereafter started dosing as per experimental design. The animals’ systolic blood pressure and pulse rate were measured weekly using the tail-cuff method on a power-lab data gathering equipment (AD Instruments). Weekly blood samples were taken from the retro-orbital plexus under chemical type (ether) anesthesia for biochemical examination [14, 15]. After completion of treatments, serum Angiotensin converting enzyme (ACE), Superoxide dismutase (SOD), Catalase (CAT), Lipid peroxidases (LPO) & Nitric oxide (NO) levels were measured.
In vitro experimental method
Estimation of Serum ACE activity 16
The synthetic substrate Hippuryl-His-Leu (HHL) was employed to test serum ACE activity. Serum was collected at the termination of the treatment schedule and mixed with HHL (5 mM) in phosphate buffered saline (NaCl 300 mM) at pH 8.3. The test and control tubes were incubated at 3700C for 30 minutes with shaking. In zero-time control tests, 1N HCl was added before the serum. The produced hippuric acid was separated from the acidified solution into the ethyl acetate form due to mixing the formed hippuric acid by action of ACE on HHL. After centrifugation, the supernatant from each ethyl acetate layer was transferred to a clean tube and heated at 12000C for 30 minutes. After then, the hippuric acid was redissolved. in 1ml distilled water and quantitative estimation was carried out by using UV-Spectrophotometer at 228nm.
Estimation of antioxidant activity:
Estimation of SOD and CAT activity 18, 17
The antioxidant activity of SOD was measured using the Kono technique, which involved inhibiting the reduction of nitroblue tetrazolium chloride by SOD (NBT). The absorbance at 560 nM by spectrophotometry. The reaction was started by mixing hydroxylamine hydrochloride with ethylenediamine tetra acetic acid (EDTA), NBT, and the post nuclear fraction of homogenate in a reaction mixture. Incubation of reaction mixture at 37℃ for 20 minutes was carried out and absorbance was taken at 560 nm. The Hydrogen peroxide breakdown was detected at 240nm in an antioxidant CAT test using the luck technique as a reference. Hydrogen peroxide phosphate buffer pH 7 was added with tissue homogenate supernatant (10%), at 240 nM, the change in absorbance was recorded after 1 minute. Millimolar extinction coefficient of hydrogen peroxide (0.071) was used to calculate the enzyme activity.
Estimation of LPO level 19
Wills’ method was used to calculate lipid peroxidation quantitatively. The amount of malondialdehyde (MDA) formed was calculated using spectrophotometry and the chemical reaction of thiobarbituric acid at 532 nm. Based on the chromophore’s molar extension coefficient, the results were reported as nanomoles (nmol) of MDA per mg of protein (1.56 x 105 M-1 cm-1)
Estimation of NO level 20
Colorimetry with Griess reagent was used to evaluate the concentration of nitrite in the supernatant, which is an indicative of the formation of nitric oxide (NO). The absorbance was measured at 543 nm using a spectrophotometer using the same volume of supernatant and Griess reagent, which was then put aside for 10 minutes in the dark. The concentration in mol of nitrite per milliliter of homogenate.
Statistical Analysis
I used Graph Pad Prism 8.0 (Graph-Pad Software: San Diego, CA, USA) to do an unpaired student t-test and a one-way analysis of variance followed by a Dunnett’s test to interpret the results. The outcome data was expressed as mean ±SEM.
Results
Table 1: Preliminary phytochemical evaluation
| Sr. No. | Test | Observation | Result |
| 1. | Carbohydrate
– Molisch test – Fehling’s test |
+ve | Carbohydrates is present |
| 2. | Test for Saponin
-Foam test |
+ve | Saponin is present. |
| 3. | Test for flavonoid
– Shinoda test |
+ve | Flavonoid is present |
| 4. | Alkaloids
– Dragendorff’s test – Wagner’s test – Mayer’s test |
+ve | Alkaloid is present. |
| 5. | Test for Phenolic Compounds | +ve | Phenolic compounds are present |
Total flavonoid and phenolic content from the U.P extract
Total flavonoid content of U. picta extract in terms of rutin equivalent was found to be 14.21 mg/g of dry weight (r2=9877). The phenolic content of U.picta extract in terms of gallic acid equivalent, 37.1 mg/g dry weight (r2=0.9966) was discovered.
Acute toxicity study
Extract of U.picta found safe up to 2000 mg/kg oral dose and fails to show any toxic effect. Rats were administered up to maximal possible dose.
Effect of U. picta extract on angiotensin II induced hypertension
Effect of U. picta extract on blood pressure (NIBP)
The group II showed significantly (**p<0.001) increased in blood pressure as compared to group I. Group V and VI showed Significantly reduced in blood pressure as compared to Positive control in experimental animals.
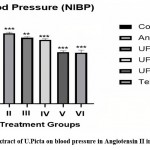 |
Figure 2: Effect of extract of U.Picta on blood pressure in Angiotensin II induced Hypertension. |
Effect on catalase levels in rat aorta
Group III showed non-significantly elevated in serum CAT level. Group IV, V and VI showed Significantly (P<0.001) increased in CAT level as compared to Group II and group III.
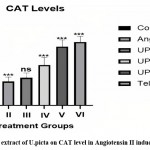 |
Figure 3: Effect of extract of U.picta on CAT level in Angiotensin II induced Hypertension |
Effect on SOD levels in rat aorta
When compared to group I, group II had a considerably lower SOD level (*p<0.05), Group IV, Group V, and Group VI demonstrated Significantly Elevated SOD levels when compared to Group II. Group III.
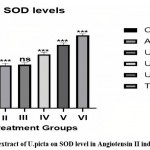 |
Figure 4: Effect of extract of U.picta on SOD level in Angiotensin II induced hypertension. |
Effect on lipid peroxidase (LPO) level in rat aorta
In comparison to group I, group II had a considerably higher amount of LPO (**P<0.001). In the experiment animal, Group III had a non-significant drop, however Group IV, Group V & VI had a considerably lower LPO level than Group II.
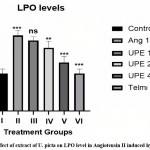 |
Figure 5: Effect of extract of U. picta on LPO level in Angiotensin II induced hypertension. |
Effect on nitric oxide (NO) level
When compared to group I, group II had a considerably lower NO level (**P<0.001) In experimental animals, Group III, Group IV, Group V, and Group VI demonstrated Significantly gradual Elevated NO levels when compared to Group II.
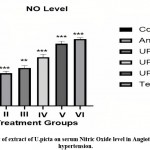 |
Figure 6: Effect of extract of U.picta on serum Nitric Oxide level in Angiotensin II induced hypertension. |
Effect on serum ACE level
When compared to group I, serum ACE levels in group II were considerably higher (**P<0.001) Serum ACE levels were significantly lowered in group V, while serum ACE levels were not significantly reduced in groups III, IV and VI.
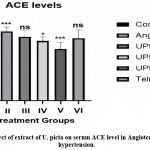 |
Figure 7: Effect of extract of U. picta on serum ACE level in Angiotensin II induced hypertension. |
When compared to group I, serum ACE levels in group II were considerably higher (P0.001).
Group I: Vehicle (distilled water 5 ml/kg BW p.o.);
Group II: Angiotensin II (150μg/kg BW i.p.);
Group III: Angiotensin II + U. picta extract low dose (100mg/kg BW, p.o.);
Group IV: Angiotensin II + U. picta extract medium dose (200mg/kg BW p.o.)
Group V: Angiotensin II + U. picta extract high dose (400mg/kg BW p.o.)
Group VI: Angiotensin II + Telmisartan (0.8mg/kg BW p.o.)
Values are expressed as mean ± S.E.M
Group II was compared with Group I
Group III, IV V and VI were compared with Group II. One- way ANOVA followed by Dunnett’s test
Discussion
Plant source of medicine are trending now a days due to less side effects. The hypertension has a number of causes of which one is the vascular endothelial dysfunction. Due to this the arteries do not work properly; contraction and relaxation of arteries does not occur properly due to the damage to the cells. This damage to the cells occurs due to elevated level of ROS in the physiology. There are various kind of anti-oxidant defense mechanism is present in the body to combat the increased oxidative stress. Different chemicals, such as free radicals, ROS, and peroxide radicals, are produced during metabolism. These molecules if not neutralized by body they can cause damage to the cells. In order to neutralize these molecules different molecules and enzymes are involved such as CAT, LPO, SOD, NO etc. When defense mechanisms are weakened then oxidative stress prevails and damage to the cells starts. In time it may increase and may result in complication such as a hypertension. One another cause of hypertension is problem in functioning of RAAS system. The ACE is responsible for converting Ang I into active Ang II (Active). Ang II stimulates the release of aldosterone (causes retention of sodium and water for maintenance of BP) and itself also a vasoconstrictor so this way it maintains blood pressure. NO levels also affect the blood pressure as it is a natural vasoconstrictor and involved in the maintenance of blood pressure in the body.
U.picta contains phenol and flavonoids along with different substances like alkaloids, glycosides, saponins, tannins etc. ACE inhibitory action has been reported in phenolic and flavonoid compounds such as quercetin, rutin, rhoifolin, caffeic acid, and chlorogenic acid, which are antioxidants in nature. Before the start of the experimental procedure the acute oral toxicity study of the plant extract was done. In that study the 2 mg/kg p.o dose (highest dose) of extract did not show any mortality or any other sign of toxicity so we can say that the plant extract is safe for use as its highest dose didn’t cause any problem.
In the ang II induced model of hypertension, the dose of 150µg/kg i.p. of ang II causes the retention of fluid due to release of aldosterone that is antidiuretic hormone that leads to hypertensive condition. The ang II leads to the increased production of O2– via the NADPH oxidase stimulation in the vessels of rats. Ang II is responsible for the increased oxidative stress levels. [36] In-vitro assay was performed for their ACE inhibition activity.
According to above performed study Group VI and group V significantly reduced ACE level compared with positive control.
Antihypertensive medicines with antioxidant characteristics appear to be the most effective treatment for hypertension to date, as they are able to lower blood pressure by influencing molecular processes involved in the control of both vascular function and oxidative state. Blood pressure evaluation is the major tool of diagnosis for antihypertensive studies.
So as per the current study we observed than group V and VI showed marked reduction in blood pressure as compare to positive control.
Catalase is a crucial enzyme that feeds on hydrogen peroxide, a nonradical ROS. This enzyme is in charge of neutralizing hydrogen peroxide by decomposing it and SOD Superoxide dismutase (SOD) play a critical role in the body’s antioxidant defense against oxidative stress. The enzyme is an effective treatment for diseases caused by reactive oxygen species. To improve its therapeutic efficiency, SOD conjugates and mimetics have been created.
In present study group IV, V and VI showed elevated level of CAT and SOD as compared to group II and III.
Because superoxide dismutase (SOD) enzymes regulate vascular levels of O2 *, a role for SOD in cardiovascular disease is of great interest. Extracellular SOD is a primary type of SOD found in the vessel wall.
As per above observation group I showed significant elevated level in negative control group and some group when compare with group IV, V and VI observed significant reduction in LPO level.
Nitric oxide (NO) is a naturally occurring chemical that can be found in a wide range of cell types and organ systems. NO is an essential determinant of baseline vascular tone in the cardiovascular system, as it prevents platelet activation, limits leukocyte adherence to the endothelium, and modulates cardiac contractility and blood pressure. We observed that group III, IV, V and VI showed gradual increased in NO level when compared with negative control.
Thus, Uraria picta Jacq. Extract treated rats showed significant antioxidant and antihypertensive activity in angiotensin induced hypertension experimental animal model from the above experimental data.
Conclusion
The plant extract has shown significant reduction in blood pressure and serum ACE level up to normal range. The toxicity study showed that the extract is safe at higher dose of 2g/kg. The plant extract has shown significant elevation in the levels of endogenous antioxidants such as GSH, catalase, peroxidase. etc. which combat the oxidative stress responsible for endothelial dysfunction and later hypertension. The plant contains different constituents including flavonoid and phenolic compound which could be responsible for its Antihypertensive activity. As a result of the current study, we can speculate that U.Picta extract may have antihypertensive properties.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
There is no funding or financial support for this research work.
References
- Ritter J. M., Lewis L. D., Mant T. G. K., Ferro A. A.: Textbook of Clinical Pharmacology and Therapeutics. 5th ed. Hodder education. London UK; 2008; pp 185-196.
CrossRef - Colledge N. R., Walker B. R., Ralston S. H.: editor. Davidson’s principles and practice of medicine. Edinburgh. Churchill Livingstone Elsevier. 2010; pp 551
- Silva G. C., Braga F. C., Lemos V. S., Cortes S. F. Potent antihypertensive effect of Hancornia speciosa leaves extract. Phytomedicine. 2016;23(2):214-9.
CrossRef - Lackland D. T., Weber M. A. Global burden of cardiovascular disease and stroke: hypertension at the core.Can J cardiopath 2015;31(5):569–71.
CrossRef - WHO A global brief on hypertension: Silent killer, global public health crisis. World health day 2013; Geneva, Switzerland: World Health Organization.
CrossRef - Seedat Y. K. Hypertension in developing nations in sub-Saharan J Hum Hypertens. 2000;14(10-11):739-47.
CrossRef - Kokate C.K., Purohit A.P., Gokhale S.B.: Pathway to Screen Phytochemical Nature of Natural Drugs. In: Text Book of Pharmacognosy. 31st Edition Nirali Prakashan. 2005; pp 593-597
- Chang C. C., Yang M.H., Wen H. M., Chern. J.C.: Estimation of total flavonoids content in Propolis by two complementary colorimetric methods. J Food Drug Anal 2002;3(10):178-182.
CrossRef - Pourmorad F., Hosseinimehr S. J., Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnology 2006;11(5): 42-1145.
- Organisation Economic for cooperation and Development (OECD), guideline for testing and chemicals 420,2001.
- Govender M. M., Nadar A. A. Subpressor Dose of Angiotensin II Elevates Blood Pressure in A Normotensive Rat Model by Oxidative Stress. Res. 2015;153-159.
CrossRef - Rajgopalan S., Kurz S., Munzel T., Harrison D. G., Freeman B. A. Angiotensin II mediated hypertension in the rat’s increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Clin. Investig.1996;97(8):1916-1923.
CrossRef - http://www.procedurewithcare.org.uk/category/oral-gavage
- Subramani P., Khor M. Z., Ramasamy R. Retro-orbital blood sample collection in rats a video Article. P T B Report. 2015;1(2):37-40.
CrossRef - Technical Library, ELISA Sample Preparation Protocols | Thermo Fisher Scientific – UK, Thermofisher.com; URL: https://www.thermofisher.com/in/en/home/references/protocols/cell-and-tissue-analysis/elisa-protocol/elisa-sample-preparation-protocols.html
- Cushman D.W., Cheung H. S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol 1971; 20: pp1637–1648.
CrossRef - Luck H., Catalase in: Bergmeyer, H.U. (editor), Methods of enzymatic analysis. Academic Press, NewYork.1971; 885–893.
CrossRef - Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186(1):189-95
CrossRef - Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99(3):667-76.
CrossRef - Beda N, Nedospasov A. A. spectrophotometric assay for nitrate in an excess of nitrite. Nitric Oxide. 2005;13(2):93-7.
CrossRef - Bhattachrya A., Datta A. K. Uraria Picta: An Overview. Med Aro Pl Sci Biotech. 2010; 4(1):1-4
- Naik K., Krishnamurthy R. Anti-inflammatory activity of methanolic extracts of Pseudarthria viscida and Uraria picta against carrageenan induced paw edema in albino rat. J Med Aromat Plants 2018;9(1):1-11.
- Olufemi A. O., Omoniyi Y. K., Akinyinka A. A., Ibitayo A.M. Mechanistic evaluation of toxicity, antinociceptive, anti-inflammatory and antioxidant actions of leaf extract of Uraria picta. LASU J Med Sci 2016;(1):1-10.
- Ghildiyal S., Gautam M. K., Joshi. V. K., Goel R. K. Anti-inflammatory activity of two classical formulations of laghupanchamula in rats. J Ayurveda Integr Med 2013;4(1):23-7.
CrossRef - Arka G., Anindita K., Ankit S., Kumar S. A., Kumar M. S. Preliminary evaluation of hepatoprotective potential of the polyherbal formulation. J Intercult Ethnopharmacol 2015;4(9):118-24.
CrossRef - Osazuwa E. O., Igboechi A. C. Anti-microbial activity of a chemical isolate from the leaves of Uraria picta. Phytother Res 1988;4(2):204-6.
CrossRef - Amole O. O., Mofomosara S. H., Ekene O. A. Evaluation of the antidiarrhoeal effect of Lannea welwitschii Hiern (Anacardiaceae) bark extract. Afr J Pharm Pharmacol 2010;4(4):151-7.
- Patel B. D., Kamariya Y. H., Patel M. B. Free radical scavenging potential of ethanolic extract of Uraria picta Pharmacologyonline. 2011;(2):134-45.
CrossRef - Odubanjo V. O., Oboh G., Ibukun E. O. Antioxidant and anticholinesterase activities of aqueous extract of Uraria picta (Jacq.) DC. Afr J Pharm Pharmacol 2013;41(7):2768-73.
CrossRef - Sonibare M. A., Ayoola I. O., Elufioye T. O. Antioxidant and acetylcholinesterase inhibitory activities of leaf extract and fractions of Albizia adianthifolia (Schumach) WF Wright. J Basic Clin Physiol Pharmacol. 2017;28(2):143-148
CrossRef - Sarris J., McIntyre E., Camfield D. A. Plant-based medicines for anxiety disorders, Part 1: a review of preclinical studies. CNS Drugs 2013;27(3):207-19.
CrossRef - De Sousa A. Herbal medicines and anxiety disorders: An overview. J Med Plants 2013; 27(3):207.
- Roy-Byrne P. P., Bystritsky A., Russo J., Craske M. G., Sherbourne C. D., Stein M. B. Use of herbal medicine in primary care patients with mood and anxiety disorders. Psychosomatics 2005;46(2):117-22.
CrossRef - UrariaPicta-DabraFlowersofindia.net https://www.flowersofindia.net/catalog/slides/Dabra.html.
- Web Design and Web Development Freelancer Website Design and Developed Chirag Kansoda (www.chiragpkansoda.com) – SEO Plant Details – Information about Uraria picta Plant, Efloraofgandhinagar.in, http://www.efloraofgandhinagar.in/herb/uraria-picta.
- Garrido A. M., Griendling, K. K. NADPH Oxidases and Angiotensin II Receptor Signaling Mol Cell Endocrino. 2009, 309(2), 148-158.
CrossRef - Erum Iqbal a, Kamariah. A. S., Linda B.L., Lim a. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of oniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. Sci. (2015) 27, 224–232.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





