How to fight the current alarming invasion of the Parthenium weed?
Parthenium genus includes a minimum of 15 described species. Today, one of the Parthenium species, the P. hysterophorus, has become a very noxious weed for many farmers in Africa and Asia. Coming from North America, this species invaded the Asian continent in the 1950s and continues to spread in new countries at an alarming rate. The plants are causing severe damage to crops and natural habitats and triggering human and cattle diseases.
What is Parthenium?
Parthenium is a genus from the family Asteraceae and subfamily Asteroideae. It is native to Mexico, USA, and Argentina, and between 14 and 33 species have been described, depending on the type of taxonomy used. Those plants are dicotyledons, annuals, biennials, perennials, subshrubs, and shrubs.
Parthenium hysterophorus L., 1753, is the most problematic species of that genus. It is a serious invasive weed species in 50 countries worldwide, mainly in the USA, Australia, Asia, and Africa (worldplants.de).
Common English names for Parthenium hysterophorus L. include: barley flower, bastard feverfew, bitter-broom, bitterweed, broomweed, carrot grass, congress grass, congress weed, dog flea weed, false camomile, false ragweed, famine weed, featherfew, feverfew, mugwort, parthenium weed, ragweed, ragweed parthenium, Santa Maria feverfew, star weed, white top, white top weed, whiteheads, wild carrot weed, wild wormwood, wormwood (inpn.mnhn.fr; CABI PlantwisePlus).
How to recognize Parthenium hysterophorus weed?
Parthenium hysterophorus is a multi-branched herbaceous annual plant. The plants can grow up to 1,5 m tall within two weeks, and some will eventually reach 2 m. The plants remain in the vegetative growth phase for 4 to 8 weeks and may flower as early as 35 days after germination (CABI Invasive Species). They stay in the reproductive stage for 12 to 16 weeks, and fruit development and seed maturation occur within 1 to 2 weeks after flowering. The plant completes its life cycle within 16 to 18 weeks (Tanveer et al., 2015).
Physiology of Parthenium hysterophorus weed
Here are some of its characteristics:
- Stems: The plant has one erect main stem that becomes woody as it matures. Mature stems are green, longitudinally grooved, and covered with fine hairs, producing many branches with flowers.
- Leaves: Young plants have leaves at their base (8-20 cm long, 4-8 cm wide) in rosettes, which are very jagged and lobed. At maturity, the newly emerging leaves are pubescent (covered with fine hairs), narrower (3-18 long, 1-9 cm wide), with leaf blades being ovate to elliptic, pinnately lobed, and less jagged: both faces (leaf sides) are sparse to densely scabrous and gland-dotted. Mature leaves are pale green, deeply lobed, arranged alternately on the stems, and are less hairy than young leaves. After flowering, most leaves die.
- Flower: Flowers are star-shaped, appearing in white to creamy-white hues, clustered at the ends of upper branches. Each flower has five petal-like ray florets, each carrying a single seed.
- Seed: dark brown to black, diamond-shaped, flattened achenes, 2 mm long with small straw-yellow wings at one end
- Root: deep taproot
(Adkins & Shabbir, 2014; Flora of China; Flora of North America; National Scientific Secretariat on Invasive Alien Species; Tanveer et al., 2015)

Pictures of Parthenium hysterophorus to help the identification
a. Rosette stage, b. entire branch and c. inflorescence of Parthenium hysterophorus. (Picture a by Indiana Coronado, from WOF – https://www.worldfloraonline.org/taxon/wfo-0000065156, pictures b and c from the online National Inventory of natural heritage (INPN) database, Muséum national d’Histoire naturelle – https://inpn.mnhn.fr/espece/cd_nom/446978/tab/fiche).
What does Parthenium hysterophorus cause? – Harmful aspects of Parthenium hysterophorus weed for the crops and human health
P. hysterophorus has been declared a noxious weed in many countries and is listed in the “Global Invasive Species Database”.
It frequently causes significant yield losses, as evidenced by some commonly used names like “famine weed”. P. hysterophorus releases allelopathic chemicals into the soil (Tanveer et al., 2015), which have been documented to inhibit the germination and growth of agronomic crops, vegetables, trees, pasture plants, and various other weed species, affecting the density and diversity of natural flora and causing changes in natural habitats such as grasslands, open woodlands, river banks, and floodplains. Those allelopathic chemicals are toxins and are a threat to several crops, including rice (Oryza sativa L.), maize (Zea mays L.), sorghum (Sorghum bicolor L.), wheat (Triticum aestivum L.), teff (Eragrostis Tef Zucc. Trotter), and a lot of other crops, as reported by CABI PlantwisePlus (Table: Host Plants and Other Plants Affected). They can reduce grain crop yield by up to 40% and forage production like buffel grass (Cenchrus ciliaris L.), sorghum fodder, and maize fodder by up to 97% (Tanveer et al., 2015; ECHO; Rwomushana et al., 2019). In India, the annual expenditure for the restoration of grazing land is around USD 6.7 billion (Rwomushana et al., 2019). P. hysterophorus pollen grains can also inhibit fruit set in tomatoes, brinjal, beans, and several other crop plants (Lalita et al., 2018).
What is parthenium allergy?
Aside from detrimental effects on agricultural production and biodiversity, it also harms human and animal health. In humans, contact with the allergens produced by the plant causes dermatitis, eye irritation, and throat infections, and its pollen causes respiratory problems (asthma and bronchitis). They can also induce dermatitis and mouth sores in domestic animals, and especially cattle. P. hysterophorus is also responsible for bitter milk: when the fodder is contaminated with P. hysterophorus leaves, animals will get digestive disorders, eat less, and their milk will be of lesser quality. The main substance that is responsible for those diseases is called “parthenin“, which is dangerously toxic. In a few extreme cases, if the animals eat the leaves in large quantities, they can die from it (CABI, ECHO; Rwomushana et al., 2019). In Queensland, Australia, the reduction of beef production and the increase of the control of the beef pasture cost farmers and pastoralists over $ 100 million a year (Adkins & Shabbir, 2014).
The current alarming situation of Parthenium hysterophorus
The expansion of the weed Parthenium weed – Where Parthenium weed can be found?
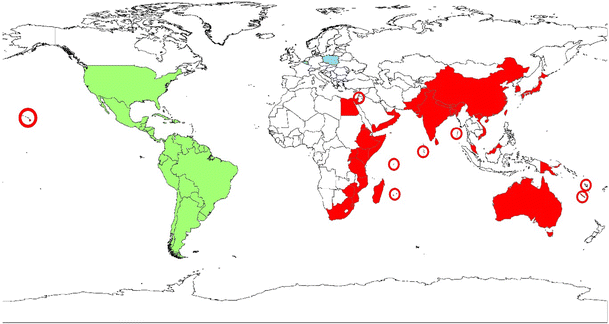
A worldwide map of parthenium weed distribution
P. hysterophorus can grow well from temperate to tropical zones and tolerate drought, cold, heat, and elevated CO2 levels (Mao et al., 2021). Its invasion started by exporting contaminated grains from Texas and Mexico to India and Australia. This species has a high plasticity and can adapt very well to new environments. The expansion of P. hysterophorus is linked to its allelopathic effects, its vigorous competition for soil moisture and nutrients, its ability to seed throughout the year, its ability to develop strong arbuscular mycorrhizal association, and its ability to outcompete the natural biodiversity in diverse habitats. It can, therefore, be found along roadsides, railwaysides, pastures, cultivated or empty farmlands, near lakes, parks, or water channels (Adkins & Shabbir, 2014; Lalita et al., 2018; Rwomushana et al., 2019). The seeds of those plants have been internationally spread via border traffic and unchecked imports, local roads and vehicles, water and wind, contaminated seed feedlots, agricultural implements, and being part of floral bouquets.
As a consequence, it is currently established or spreading over Australia (especially in the pastoral regions of Queensland), Asia (mainly India, Pakistan, Bangladesh, Nepal, Vietnam, China and Sri Lanka), East Africa (especially in South Africa, Ethiopia, Kenya, Swaziland, Mozambique, Zimbabwe, Mauritius, Madagascar, Uganda and now in Northern Tanzania). It is also present in several Latin American and Caribbean countries but with less disturbance than in the other mentioned parts of the world (CABI; ECHO; Tanveer et al., 2015; Lalita et al., 2018; Rwomushana et al., 2019 – see map).
The invasive nature of this species is concerning; its importation or sale is prohibited in the European Union (European Commission, 2021). Australia imposed strict quarantine on contaminated equipment and stock, and any plant found shall be reported/controlled (Adkins & Shabbir, 2014; Rwomushana et al., 2019), and the Suppression of Noxious Weeds Act of 2010 (CAP 325) obliges landowners in Kenya to remove the plant from their lands (Rubaba et al., 2017).
Parthenium hysterophorus (Parthenium weed) management strategies
How to prevent Parthenium hysterophorus from invading more lands?
Prevention strategies:
The strong invasiveness of P. hysterophorus is mainly due to its high and all-year-round seed production. An average plant produces 15,000 seeds, and large plants are known to produce 100,000 seeds (Global Invasive Species Database).
Here is a list of major measures to prevent Parthenium’s propagation:
- Quarantine measures to check for the introduction of this weed from infested to non-infested areas (transportation of consumer goods, movement of livestock, etc.)
- Monitoring after favorable weather conditions for its seeds’ dispersal (strong wind, flooding, etc).
- Continuous removal of the weed should be practiced to exhaust the soil seed bank completely. Indeed, P. hysterophorus can produce an enormous seed bank, representing up to 50% of the total soil seed bank (Senseman & Oliver, 1993). Furthermore, if the floor is undisturbed, buried seeds can stay dormant for up to six years (Lalita et al., 2018).
- Root out any plants that are found before the plants set seeds. The best is to remove them before flowering to avoid pollen production (causes allergies).
Caution! Removing the weed manually without protective clothing poses a significant risk of dermatitis!
- Educate the general public and farmers in its identification and elimination via broad publicity campaigns through radio, TV, posters, and seminars campaigns, like it had been done in Ethiopia or in Pakistan (Rwomushana et al., 2019; CABI)
Manual or mechanical weeding of Parthenium hysterophorus:
- Manual uprooting and hoeing of the plants before flowering and seed production, followed by sowing desirable crops or pasture species, can be helpful. Uprooting the plant is necessary, as it can regenerate and regrow from cut or partly pulled plants with a root system.
- Remove weeds in uncultivated fields to prevent the weeds from facilitating the survival of pests.
Caution! Removing the weed manually without protective clothing poses a significant risk of dermatitis!
- Mechanical treatments (grading, mowing, slashing, and plowing) after the plants set pollens or seeds are inappropriate since they may promote seed dispersal.
(CABI PlantwisePlus)
Chemical control of Parthenium – Using Herbicides to control Parthenium hysterophorus:
Parthenium is more sensitive to herbicides at the active vegetative stage than flowering stage. Tanveer et al. (2015) and Lalita et al. (2018) reported the most effective pre-and post-emergence herbicides for the control of P. hysterophorus in cropped and non-cropped areas. 
Parthenium rosette. Picture source: https://www.agric.wa.gov.au/declared-plants/parthenium-weed-declared-pest
Herbicides that could be used in cropped areas as pre-emergence applications are: oxadiazon, oxyfluorfen, butachlor and pretilachlor in upland drilled rice. Those that could be used as post-emergence applications are: 2,4-D, atrazine and pendimethalin in maize and pearl millet (Pennisetum typhoides L.), clomazone in soybean fields, atrazine in sugarcane ratoon, glyphosate, 2,4-D Na salt and metsulfuron methyl were quite effective in controlling parthenium in pastures and grasslands, atrazine and metribuzin in sugarcane. A mix of bromoxynil + MCPA could be used to manage the weed in cropped areas (Ali et al., 2020), and an application of pendimethalin followed by bromoxynil may provide acceptable control (>85%) of the weed in sorghum fields (Bajwa et al., 2023).
Those that could be used in non-cropped areas as pre-emergence applications are: terbutryne, atrazine, simazine, butachlor, dimethyl amine salt, ethyl ester and sodium salt of 2,4-D, penoxalin, benthiocarb, methabenzthiazuron and bentazone. Those that could be used as post-emergence applications are: mono-sodium methyl arsenate (MSMA), diquat, glyphosate, 2,4-D, dicamba, picloram, diuron chlorimuron ethyl, metsulfuron methyl, atrazine, metribuzin, and glufosinate.
Tolerance to herbicides was observed to: paraquat, glyphosate, flazasulfuron, fomesafen, and glufosinate (Njoroge, 1991; Heap, 2017; Palma-Bautista et al., 2020). For the glyphosate, it has been reported that elevated CO2 can delay its action (Bajwa et al., 2019).
Caution! ALWAYS read and follow the instructions on the product label and the local guidelines! Farmers are advised to consult a local licensed agronomist or a relevant authority (extension center) before applying ant chemical control.
Bioherbicides for P. hysterophorus management
P. hysterophorus can also be controlled by bioherbicides. Those present the advantage of minimally affecting the environment and human health.
The allelopathic potential of extracts, residues, and essential oils of many plant species (Cassia, Amaranthus, Xanthium species, Desmodium uncinatum), grasses (Imperata cylindrica, Desmostachya bipinnata, Otcantium annulatum, Sorghum halepense, Dicanthium annulatum, Cenchrus pennisetiformis), and trees (Eucalyptus tereticornis, Azadirachta indica, Aegle marmelos, Mangifera species, etc.) have demonstrated inhibitory activities on seed germination and seedling growth of P. hysterophorus (Kaur et al., 2014; Tanveer et al., 2015; Ojija et al., 2019).
Additionally, metabolites and filtrates of several fungi (Fusarium oxysporun and Fusarium monilifonne, Colletotrichum, Curvularia, Myrothecium, Sclerotium rolfsii, Phoma herbarum, Alternaria japonica and Alternaria macrospora) exhibit bioherbicidal activity against the seeds and/or the seedlings of this weed and are considered as mycoherbicidal agents (Vikrant, et al., 2006; Singh et al., 2013; Tanveer et al., 2015; Kaur et al., 2015; Javaid et al., 2017; Kaur and Aggarwal, 2017); CABI PlantwisePlus).

Parthenium weed flowering
Displacement of the weed by competition:
The “displacement by competition” technique is easily implementable, sustainable over time, profitable across various environmental conditions, and helps the biodiversity of native plants. Various studies revealed that growing certain fodder species like Cassia spp., Tagetes spp., Bothriochloa insculpta cv. Bissat (Creeping bluegrass), Dichanthium aristatum, Poa pratensis, Cenchrus ciliaris, Clitorea terneata cv. Milgarra, Chloris gayana, Croton bonplandianus, Amaranthus spinosus, Tephrosia purpurea, Hyptis suaveolens (Mesosphaerum suaveolens), Kochea indica, Sida spinosa, and Mirabilis jalapa is a way to suppress the growth of P. hysterophorus. These fodder species produce allelopathic molecules, can outcompete the weed by having a higher assimilation rate, and cast shade on the weed. The weed plant growth is reduced, as well as its seed production (Donnell & Adkins, 2005; Kaur et al., 2014; Khan et al., 2014; Tanveer et al., 2015).
Biological control of P. hysterophorus weeds – Biological control agents
Biological control implicates living organisms that can cause diseases or directly eat parts of the plant, often killing the weed and/or reducing its seed production. The aim is to maintain the weed population below a certain threshold while minimally impacting native plants or crops. Using biological agents such as arthropods, insects, and microbial pathogens is an efficient, long-term, affordable, practical, and sustainable solution to fight against P. hysterophorus.
Biological control methods for P. hysterophorus involves the use of:
1. Arthropods and insects, such as:
- the leaf-feeding Mexican beetle Zygogramma bicolorata Pallister,
- the seed-feeding weevil Smicronyx lutulentus Dietz.,
- the stem-galling moth Epiblema strenuana Walker,
- the leaf-mining moth Bucculatrix parthenica Bradley,
- the sap-feeding plant hopper Stobaera concinna Stal,
- the stem-boring curculionid weevil Listronotus setosipennis Hustache,
- the stem-galling weevil Conotrachelus albocinereus Fiedler,
- the root-boring moth Carmenta sp. nr. ithacae (Beutenmuller)
- the moth Platphalonidia mystica Razowski & Becker,
- several mealybug species
2. Pathogenic organisms (via spore suspension and culture filtrates):
- the soil fungi Fusarium oxysporum and Fusarium moniliforame,
- the rust fungi Puccinia abrupta Dietand Holw. Var. partheniicola (winter rust) and Puccinia xanthii Schwein. var. parthenii-hysterophorae (summer rust),
- the bacterial strain of Xanthomonas campestris pv. Parthenii,
- the phytoplasma bacteria of faba bean phyllody group (FBP).
While the effectiveness of biological control measures has been proven, its success depends a lot on factors linked to the life cycle synchronization between the agent and the host, its adaptation to the local environment, climatic conditions and altitude, the cultural practices, and other pest management strategies put into place. Finally, a lot of living organisms proven their efficacy on the management of the weed plant, but it is still needed to conduct more research programs to find natural P. hysterophorus seed-feeding agents to deplete its seed bank in the soil (Adkins & Shabbir, 2014; Tanveer et al., 2015; Maharjan et al., 2019; Rwomushana et al., 2019; Iqbal et al., 2020; CABI PlantwisePlus).
Integrated management practices of P. hysterophorus
Combining two or several of the above-listed approaches, like the use of synthetic herbicides, bioherbicides, and biological control agents, together with certain cultural practices such as the use of straw mulch, intercropping or the displacement by competitive plant species, are reported as possible strategies for a significant reduction in P. hysterophorus growth and consequent increase in crop yield (Tanveer et al., 2015; Rwomushana et al., 2019).
Conclusion
Parthenium hysterophorus has become a major threat to both cultivated and uncultivated areas worldwide. Addressing the challenge of managing this invasive weed requires a coordinated global effort at local and regional levels to raise awareness in the general population about the health issues of the weed and how to prevent its spread, especially teaching about preventive strategies. For farmers and collectivities, several solutions exist for suppressing P. hysterophorus: manual or mechanical weeding, conventional herbicides, bioherbicides, biological agents, and displacement by competition, but an appropriate integration of several of these approaches is necessary to minimize the spread of P. hysterophorus and provide sustainable weed management.
References
Adkins & Shabbir, 2014. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Management Science.
Adriaens et al., 2020. Fausse camomille. Parthenium hysterophorus (Parthenium lobatum).
National Scientific Secretariat on Invasive Alien Species.
(https://www.iasregulation.be›download)
Ali et al., 2020. Comparative Efficacy of Common Broad Leaf Herbicides against an Invasive Weed: Parthenium hysterophorus L. Agricultural Sciences.
Bajwa et al., 2019. Effect of elevated carbon dioxide concentration on growth, productivity and glyphosate response of parthenium weed (Parthenium hysterophorus L.). Pest Management Science.
Bajwa et al., 2023. Herbicide Program to Control Parthenium hysterophorus in Grain Sorghum in an Arid Environment. Crops.
Bajwa et al., 2016. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds.” Planta 244 (2016): 39-57.
Donnell and Adkins, 2005. Management of parthenium weed through competitive displacement with beneficial plants. Weed Biology and Management.
Iqbal et al., 2020. The first record of Puccinia abrupta var. partheniicola, on Parthenium hysterophorus an invasive alien plant species in Pakistan. BioInvasions Records
Javaid et al., 2017. Management of parthenium weed using metabolites of Alternaria japonica. Planta Daninha
Kaur et al., 2014. Effects and Management of Parthenium hysterophorus: A Weed of Global Significance. International Scholarly Research Notices.
Kaur et al., 2015. Screening of phytotoxicity of Alternaria macrospora MKP1 against Parthenium hysterophorus L.. Archives of Phytopathology and Plant Protection.
Kaur and Aggarwal, 2017. Biocontrol potential of Alternaria sp. PMK2, against a devastating weed: Parthenium hysterophorus L. International Journal of Pest Management
Khan, et al., 2014. Suppressive fodder plants as part of an integrated management program for Parthenium hysterophorus L.. Field Crops Research.
Lalita et al., 2018. Review on a weed Parthenium hysterophorus (L.). Int J Cur Res Rev.
Maharjan et al., 2019. Temporal and spatial patterns of research on a globally significant invasive weed Parthenium hysterophorus L.: A bibliographic review. Crop Protection.
Mao et al., 2021. A superweed in the making: adaptations of Parthenium hysterophorus to a changing climate. A review. Agronomy.
Njoroge, 1991. Tolerance of Bidens pilosa L. and Parthenium hysterophorus L. to paraquat (Gramoxone) in Kenya coffee. Kenya Coffee.
Ojija et al., 2019. Bio-herbicide potential of naturalised Desmodium uncinatum crude leaf extract against the invasive plant species Parthenium hysterophorus. Biological Invasions.
Palma-Bautista et al., 2020. Evolving Multiple Resistance to EPSPS, GS, ALS, PSI, PPO, and Synthetic Auxin Herbicides in Dominican Republic Parthenium hysterophorus Populations. A Physiological and Biochemical Study. Agronomy.
Rubaba et al., 2017. Scope of research on Parthenium hysterophorus in Africa. South African Journal of Plant and Soil
Rwomushana et al., 2019. Parthenium: Impacts and coping strategies in Central West Asia Evidence Note (March 2019). KNOWLEDGE FOR LIFE, CABI.
(https://www.invasive-species.org/wp-content/uploads/sites/2/2019/04/Parthenium-Evidence-note_FINAL.pdf)
Senseman and Oliver, 1993. Flowering patterns, seed production, and somatic polymorphism of three weed species. Weed Science.
Singh et al., 2013. Evaluation of toxins of phytopathogenic fungus for eco-friendly management of Parthenium. Indian Journal of Weed Science.
Tanveer et al., 2015. Interference and management of parthenium: The world’s most important invasive weed. Crop Protection.
Vikrant, et al., 2006. Characterization of a Phytotoxin from Phoma herbarum for Management of Parthenium hysterophorus L.. Journal of Phytopathology.
Websites:
CABI. Rooting out parthenium weed in Pakistan. https://www.cabi.org/stories-of-impact/rooting-out-parthenium-weed-in-pakistan/
CABI Invasive Species. Parthenium in Pakistan. Video
https://www.invasive-species.org/species/parthenium/
CABI PlantwisePlus. Parthenium hysterophorus (parthenium weed).
https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.species.45573
ECHO. Parthenium hysterophorus – Video. echo community.
https://www.echocommunity.org/fr/resources/3e4b4a13-3a1b-4031-831c-dd07d55bd46d
European Commission. EU Regulation 1143/2014: assessment of invasive alien species of Union concern distribution. JRC Publications Repository (202
https://publications.jrc.ec.europa.eu/repository/handle/JRC123170
Global invasive species database. Parthenium hysterophorus.
https://www.iucngisd.org/gisd/species.php?sc=153
Flora of China. Parthenium hysterophorus Linnaeus.
http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200024340
Flora of North America. Parthenium hysterophorus Linnaeus.
http://floranorthamerica.org/Parthenium_hysterophorus
Heap, 2017. The International Survey of Herbicide Resistant Weeds.
http://www.weedscience.orgPicture a comes from: WFO. Parthenium hysterophorus L. http://www.worldfloraonline.org/taxon/wfo-0000065156
Picture b and d comes from: MNHN & OFB [Ed]. 2003-2023. National inventory of natural heritage (INPN). Parthenium hysterophorus L., 1753.










































































