Abstract
Crops that offer multiple types of value, for example, in terms of economy, culture, and health, frequently lack information on the basic ecological interactions that play a significant role in their persistence. Chayote (Sechium edule, Cucurbitaceae), a widespread squash valued for its nutritious fruit, is one example. We aimed at describing the assemblage of flower visitors to this crop at 2400 m where stingless bees, reported as the main pollinators, are naturally absent. We implemented flower exclusions, performed field observations, and inspection of pollen loads of captured visitors to be able to draw distinctions between primary and secondary pollinators. We recorded a total of 60 species or morphospecies of insects visiting the flowers. Bees, wasps, and flies, present in all sites and consistently carrying abundant pollen, are the main pollinators of S. edule at high altitudes. Our study adds to the building evidence of the fundamental role that native bees and wasps play as crop pollinators in subsistence farming. Basic ecological knowledge is essential to inform agricultural management policies and to foresee preventable food scarcity problems, especially in view of climate change scenarios that predict drastic alterations in plant geographical distributions.
Similar content being viewed by others
1 Introduction
The crucial role pollinators play in maintaining the equilibrium of natural and agricultural systems (IPBES 2019) is intrinsically related to the achievement of food sovereignty (Benítez et al. 2014) by maximizing contributions of ecosystem services (Pimbert 2018). Additionally, the economic value of pollination services in favor of agriculture, performed by biological agents, has been estimated to be over 400bn USD every year (IPBES 2016). Humans strongly rely on a small percentage of edible plants (Warren 2015) grown all around the world, and in some instances, these same crops are crucial for medical advances (Panda et al. 2011; Yeo et al. 2021). However, a good understanding of the ecological interactions of a crop, which can enhance production and be a driver for its success or even ensure its existence, is more often than expected missing from the published literature. This is the case for Sechium edule (Jacq.) Sw., a commercial vegetable with a handful of publications mentioning some aspect of its pollination biology in contrast to a vast body of literature pointing towards the importance of this plant in areas such as nutrition (Vieira et al. 2019), health (researched for its properties to fight cancer cells; Salazar-Aguilar et al. 2017), economy (Cadena Iñiguez and Arévalo Galarza 2010), and cultural value (Almaguer González et al. 2020 and references therein).
Sechium edule is a Cucurbitaceae, called chayote or hüisquil in Mexico and Guatemala (respectively), vegetable pear or choko in English, that was presumably domesticated prior to the sixteenth century in pre-Columbian times, by the Mexica in central Mexico and Guatemala (Newstrom 1986). Presently, this crop is cultivated, for example, in North and South America, Southern Asia, Australia, and New Zealand (Rojas-Sandoval 2018), in addition to areas where it is naturally distributed in tropical and subtropical regions of Mesoamerica and the Caribbean. It is grown at altitudinal ranges from sea level to above 3000 m (González-Santos et al. 2017). Chayote is a crop of cultural, economic, and nutritious value in Mexico (Cadena Iñiguez and Arévalo Galarza 2010 and references therein; Almaguer González et al. 2020). It is reported to be pollinated by Apis mellifera L. (Somá Álvarez and Núñez Grajales 2013) but especially by stingless bees such as Nannotrigna perilampoides Cresson (Roubik et al. 1991) and various species of Trigona as well as some wasps (though the later are regarded as unimportant) (Heard 1999). To the best of our knowledge, published studies in relation to S. edule pollination biology report findings from elevations below 2000 m coinciding with the altitudinal distribution of stingless bees (Arnold et al. 2018). Nevertheless, since chayote is grown in highlands surpassing altitudes of 2000 m, it brings into question whether other bees or insects play a primary role in maintaining the production of this crop where stingless bees are naturally absent.
To fill in the gap of basic pollination ecology knowledge in relation to chayote cultivated at high elevations, in this paper, we report an observational and experimental study on the richness of flower visitors and pollinators. Our aim was to determine the identity and hierarchy of S. edule pollinators, within agroecological parcels located at altitudes between 2400 and 2500 m. We implemented flower exclusions, performed field observations, and inspection of pollen loads to be able to draw distinctions among primary and secondary pollinators and flower visitors. Since native stingless bees, specifically Trigona species, reported as primary pollinators for chayote are absent from the studied area, we asked whether other native bees or wasps would take on the role of principal pollinators.
2 Materials and methods
2.1 Study species
Sechium edule varieties (> 15) are all monoic one-seeded edible squashes (Cucurbitaceae) with one or two axilary pistilate flowers at the base of staminate raceme inflorescences (Fig. 1). The plant is mainly cross-pollinated but self-compatible and attracts a variety of flower visitors, thus is classified as having a generalist pollination syndrome (Newstrom 1986). The rich spectrum in terms of phenotypic plasticity and genotypic pool is reflected in fruit types varying in size, shape, color, surface, and taste (Lira et al. 1999). Sechium edule is a long-lived perennial tuber that grows abundant climbing vines forming a dense foliage cover producing hundreds of hanging fruits in a single season (Figure 1). It is cultivated by sowing the viviparous seeds, and some plants are said to be more than 20 years old (pers. com. from local producers; Figure 1). Chayote, as it is commonly known, is grown at wide altitudinal ranges (0 to 3500 m) for self-consumption by small-scale farmers in rural areas (Altieri et al. 1987; Boege 2008; Guevara Hernández et al. 2015), and when the plant produces an excess of fruits or shoots, they are sold in local markets. Notwithstanding, chayote is also grown for the sole purpose of generating income through its sale, by both small and large-scale farmers.
Sechium edule flowers and plants. A Staminate flower. B Polistes instabilis feeding on nectar from a pistilate flower. C Sechium edule plant more than 20 years old (according to the producer) that can surpass 100 m2 in cover area during a single flowering season. D Section of S. edule plant included in this study from site 3, Santa Anita. E Apis mellifera and Agelaia sp1 feeding on nectar from staminate flowers and a developing fruit at the back (left). F Mature chayote fruit
2.2 Study sites
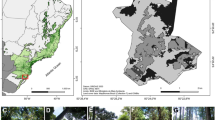
We selected five territories (Alcanfores, Vista Hermosa, Santa Anita, El Pinar, and La Florecilla) within the Jovel Basin, also known as San Cristóbal de Las Casas, located in los Altos de Chiapas (Chiapas Highlands), southeastern Mexico, where small-scale agroecological and traditional farming is practiced (milpa and home gardens). The Jovel Basin is characterized by a temperate sub-humid climate with rainy season (May–Oct) during summer and occasional winter frost during the dry season (Nov–Apr). The mean annual temperature is 14.4 °C and annual rainfall varies between 1000 and 2500 mm (INEGI 2020). We were able to work with seven small-scale farmers at seven sites (Figure 2) located above 2400 m in altitude. Selected S. edule plants were approximately 4 years old (on average), with minimum area coverage of 10 m2 (growing on trees or improvised trellis) and abundant flower buds. Bigger chayote plants have more flowers, and thus, we used plant size (as a proxy to attractiveness) to explain potential differences in visitation rates (more or different flower visitors) among plants. At each site, from one chayote plant, we selected 10 branches bearing only leaves and flower buds in which we followed the vegetative and reproductive development (recording branch length, number of leaves, presence of flower buds, and presence of developing fruits). We recorded the same variables in subsequent visits to estimate growth and productivity. On the same marked branches, we selected a flower bud to be partially excluded from large pollinators such as Apis mellifera and Bombus sp. to test the relative importance of these pollinators. To impede contact of large pollinators with stigmas, the exclusion was made by combining metal mesh of 3.5 × 3.5 mm aperture (smaller aperture than pollen traps; (Gurini et al. 2020)) and compact textile mesh (to attach to branches without damaging tissue) (Figure 3). Once excluded buds had opened and corollas were shed, we removed exclusions to allow potential fruit growth.
2.3 Floral visitors
We aimed to record the diversity of chayote floral visitors through the collection of observational data and sampling of flower visitors, during the months of October and November 2019 (end of the rainy season). To avoid biases, we assumed that every flower visitor had the potential to be a true pollinator and thus recorded visitation frequency and inspected pollen loads present on the bodies of collected specimens regardless of their identity. On 2 × 2 m survey squares, we recorded the activity of flower visitors during periods of 15 min from 9:00 to 14:00, moving plots after each period and interspacing flower visitor sampling efforts at the same plant. The number of observations and sampling efforts per day were determined by the start of rain of each sampling day (on average four observation and four sampling periods a day per site). Every plant (N = 7) was assessed for three days or until achieving a minimum of 195 min of observation without new records of flower visitor species. We considered a floral visitor as any insect that directly contacted anthers or was feeding on nectar, assigning its taxonomic identity to one of five groups: beetles, flies, native bees, honeybees (A. mellifera), and wasps, and later identified them, when possible, to species level. To identify species and assess which flower visitors are efficacious pollinators, during the interspaced sampling efforts, we collected individuals who were seen visiting flowers, following methods described in Kearns and Inouye (1993). Nevertheless, since chayote shoots are fragile and the flowers are mostly present under leaves, the capture of insects proved to be difficult and time consuming. Collected specimens were scrutinized under a dissecting microscope to determine the presence of pollen grains. Not all insects were collected directly after visiting a staminate flower, and groups of insects were simultaneously sacrificed in the same killing jar; therefore, the presence of more than 20 discernible pollen grains was used as a threshold to differentiate between jar contamination and true pollen load (Online Resource Figure 1). Collected specimens were identified by Philippe Sagot and Benigno Gómez Gómez at ECOSUR San Cristóbal.
2.4 Flower visitor pollen load
We removed all pollen from specimens following the chemical washing and mounting methods described in Caccavari and Cilla (2010) and identified plant species present in pollen loads from beetles, flies, true bugs, native bees, honeybees, and wasps. We recorded the total abundance of each pollen grain species on a DAFOR scale (Dominant ≥ 50 grains, Abundant 20 to 50, Frequent 10 to 20, Occasional 3 to 10 and Rare 1 to 3 grains) as a proxy to determine pollen transport efficiency. In order to identify the plant species present in pollen loads, we collected flower buds, herbarium samples, and took photographs from all flowering plants near or within the crop area (radius of 500 m from focal plants). The unopened (mature or about to open) sampled flower buds of every flowering plant at each site were used to create a local pollen reference library.
2.5 Statistical analysis
We wished to identify possible differences in visitation rates among flower visitor assemblages and among sites, but we lack a comprehensive dataset on flower visitor diversity with corresponding visitation rates for each studied site. Therefore, we fitted a linear mixed effects model using the function glmer.nb (negative binomial distribution) from the package lme4 v1.1–23 (Bates et al. 2012), using visitation rate (visits/h) as the response variable, insect identity, and plant size (as a proxy for number of flowers and individual plant attractiveness) as explanatory variables, and site as a random factor. The variable plant size was standardized using the function scale from the package standardize v0.2.1 to reduce multicollinearity. We fitted a full model with interactions.
To help differentiate true pollinators from flower visitors, on top of visitation rates, we replaced the qualitative DAFOR scale with median values based on pollen counts on each specimen slide (Online Resource 2) and calculated the median value of pollen abundance for those visitors with several individuals sampled (pooled among sites). In this way, we are able to differentiate between insects carrying large amounts of S. edule pollen (DA, above 20 pollen grains) and those with fewer grains (FOR, fewer than 20 pollen grains) and gain more evidence for their relative importance as pollinators.
Finally, to explain whether fruit set is influenced by visitation rate and identity of pollinators, we fitted a generalized linear model using the function glm from the package lme4 v1.1–23 (Bates et al. 2012). We calculated fruit set (fruit/m2) (response variable) and used visitation rate (visit/hour/m2) and pollinator identity as the explanatory variables. We discarded plant size based on model selection, and only used data from those pollinators that carried generous amounts of S. edule pollen (based on our DAFOR analysis) and from which we have records of visitation rates (six pollinator species) for this analysis.
All graphics were done using the package ggplot2 (functions: ggplot) (Wickham 2009), and analyses were performed using R version 4.0.3 (R Core Team 2013).
3 Results
3.1 Flower visitors
The activity of flower visitors commenced when the daily average temperature reached a minimum of 12 °C (around 08:30). Temperatures increased up to 22–24 °C (depending on the month) especially during midday and then after 17:00 quickly dropped until reaching 8–5 °C during the night (varying in relation to the proximity of the northern hemisphere winter). All visitors showed a clear preference towards nectar and did not seem to prefer male over female flowers. Within the taxonomic diversity, across all sites, we registered a total of 60 species or morphospecies from four insect orders, visiting chayote flowers, varying in composition or visitor assemblage among sites. We identified 18 species from eight families of Diptera, 31 species from five families of Hymenoptera, eight species of three families of Coleoptera and three morphospecies of Hemiptera (Table I).
Flower visitors present in all sites and consistent S. edule pollen carriers were A. mellifera, Bombus ephippiatus, Polybia diguetana, Polistes instabilis, Polybia sp. 1, Vespula squamosa, and Diptera msp. 2 (Table I; Online Resource 1 Figure 2). It is likely that other widely distributed species of Hymenoptera and Diptera carrying S. edule pollen might be present in all sites, but we are unable to confirm this, at present time, due to the difficulty in trapping insects without harming flowers or plants.
3.2 Visitation rates
We analyzed visitation rates for 15 flower visitors among our sites and found significant differences in visitation rates (X2(14, 268) = 132.15, p < 0.001) as well as in the interaction between the identity of the visitor and the size of the plant (X2(11, 268) = 23.20, p = 0.016) (Online Resource 1 Table I). The significance of the interaction indicates that visitation rates for Polybia diguetana (z = − 3.585, p < 0.001), Vespula squamosa (z = − 3.203, p < 0.001), and Agelaia sp1 (z = − 2.511, p = 0.01) varied based on the size of the plant. When average visits by A. mellifera were the highest (41 h−1 and 38 h−1) on plant sizes 64 m2 and 20 m2 (respectively), average visits by wasps were at least four times lower (average fewer than 7 h−1 and 2 h−1). In contrast, plants with fewer visits by A. mellifera (3.7 h−1, 7.7 h−1, and 1.4 h−1; plant sizes 22 m2, 30 m2, and 50 m2, respectively) had more visits by V. squamosa, Agelaia sp. 1, and P. instabilis (Online Resource 1 Table II). Interestingly, in the case of the plant with a size of 30 m2, or medium-sized plant, visitation by B. ephippiatus was similar to A. mellifera and visits by the aforementioned wasps were also high (compared to other plant sizes), suggesting a more equitable pollinator assemblage, and the presence of competition for floral resources and fewer dominant species (Figures 4 and 5; Online Resource 1 Table II). Nevertheless, the data lack a consistent pattern to demonstrate evidence for either competition among visitors or to add evidence to the hypothesis of bigger and showier plants (thus more attractive) receiving more visits. In other words, this significant interaction effect might not be biologically relevant.
Visitation rates of pollinators with a dominant (Apis mellifera, Bombus ephippiatus, Agelaia sp. 1, Polistes instabilis, Vespula squamosa) or abundant (Polybia sp. 1) Sechium edule pollen load by plant size. Big dots show means, upper and lower lines represent 95% confidence intervals, and smaller dots are the individual observation periods for each plant. Note that we excluded one data point from Apis mellifera (104 visits/h, plant 64 m2) to improve visualization and interpretation of this figure
3.3 Pollen load
Following a conservative criterion to avoid overestimation of plant species present in pollen loads, 50 morphotypes were not taken into account in our analysis. The criterion excludes rare morphotypes (one to three grains) present in a single specimen (mostly from A. mellifera slides). Therefore, we registered a total of 109 pollen species or morphotypes; we were able to identify 17 families and 61 species or morphospecies, and the rest (48 morphotypes) are undetermined plant species pollen grains (NA_ID). The most abundant family within the assessed pollen loads was Asteraceae (Compositae) with 28 pollen morphotypes, followed by Solanaceae (four morphotypes) and Cucurbitaceae (three morphotypes, including S. edule) (Online Resource 1 Table III).
We identified 60 species of flower visitors, of which 53% of bees, 56% of wasps, 39% of flies, and 25% of beetles carried S. edule pollen (in at least one individual of each species), whereas from the three collected true bugs (Hemiptera), only one had fewer than 10 pollen grains present on their body (Table I). In terms of the maximum diversity of plant species visited by flower visitors (diet breadth) in this study, the individuals of A. mellifera visited 48 flowering plants, followed by V. squamosa and B. ephippiatus (35 and 30, respectively) whereas the minimum amount was three plant species collected by individuals of Zethus sp. 1 and Diptera msp. 5 (Online Resource 2).
The highest amount of pollen species present in flower visitor’s individual pollen loads was 15 in A. mellifera and 14 in B. weisi and Crabronini sp. 1. On the other hand, the minimum count of pollen species observed on a single specimen was three for Lasioglossum costale, Zethus sp. 1 and Diptera msp. 5 and two for Chillicola sp. and Protandrena sp. 2 (Online Resource 2). Care must be taken when interpreting these results since only one individual represented several inspected species (e.g., Chillicola sp., B. weisi, or Lasioglossum costale) (Table I).
From the pool of collected flower visitors and after inspection of the pollen loads, we discerned among those that carry dominant or abundant (DA) S. edule pollen grains from those that carried frequently, occasionally or rarely (FOR, overall fewer than 20 grains), to better inform our analysis of visitation rates and hierarchy of pollinators. In this sense, the principal assembly of pollinators for S. edule at high altitudes (Jovel Basin) are A. mellifera (dominant and present in all sites), Agelaia sp 1 (dominant), B. ephippiatus (dominant and present in all sites), P. instabilis (dominant and present in all sites), V. squamosa (dominant and present in all sites), Polybia msp 1 (abundant and present in all sites), and P. diguetana (abundant) (Table I).
3.4 Fruit set
Our partial exclusion experiment of female flower buds yielded interesting results. From 63 partially excluded buds, 18 started developing fruits, but during maturation (after 3 weeks) the majority were aborted except for three fruits each from independent plants (at PI, AL, and VH). In other words, the exclusion of large pollinators, such as A. mellifera and B. ephippiatus, substantially reduced the proportion of flowers setting fruit in S. edule, a predominantly cross-pollinated and self-compatible perennial vine grown by seed sowing. We observed other flower visitors entering the partial exclusions (wasps and beetles); therefore, further and refined exclusion experiments are needed to state confidently whether visits by A. mellifera and B. ephippiatus are absolutely imperative for fruit production.
Fruit set is not significantly influenced solely by visitation rate of the selected hierarchically important pollinators (X2(1, 247) = 0.52, p = 0.46) but rather our data show significant effects from visitor’s identity (X2(5, 247) = 20.796, p < 0.001) as well as the interaction between visitation rate and visitor’s identity (X2(5, 247) = 13.93, p < 0.01) for B. ephippiatus (z = 2.385, p < 0.01) and V. squamosa (z = 2.577, p < 0.01) (Online Resource 1 Table IV).
4 Discussion
Bees are a group of fundamental pollinating insects for wild plants (Potts et al. 2010) and crops alike (Klein et al. 2007). Our study adds to this body of knowledge, demonstrating that A. mellifera and B. ephippiatus are S. edule pollinators, but also wasps such as Polybia species, Polistes instabilis and V. squamosa. All of the aforementioned insects were found at all sites, carrying consistently large amounts of chayote pollen, and they showed high visitation rates (though varying among sites) and should therefore be considered as primary pollinators. Our results sharply contradict what Wille and Orozco (1983) state as Trigona species being the sole pollinators for chayote, emphasizing that Bombus species do not visit the flowers and honeybees rarely visit them (thus classifying them as secondary pollinators). A caveat we find for their study is that they do not specify how they determined both the efficacy and capacity for pollen transportation. Additionally, their assessments excluded altitudes above 2000 m, where stingless bees are rarely distributed (Arnold et al. 2018). On the other hand, the same authors report a comprehensive list of secondary pollinators, which include several species that we found to be visiting the plant, transporting copious amounts of S. edule pollen and present in three or more sites, such as Lasioglossum species and Polybia species (including Polybia diguetana). We thus encourage the revision of long-accepted premises in pollination studies, to vindicate the role of unpopular pollinating insects as part of agroecosystems.
A hierarchy of pollinators is informative, adds to the building evidence of the key role that bees play, but at least for chayote pollination, we demonstrate that wasps play a primary role and should not be thought of merely flower visitors (Brock et al. 2021). In addition, we must keep in mind that other visitors, such as flies, are not less important pollen carriers (Rader et al. 2015), and thus, we hereby categorize them as insurance pollinators (Martínez-Bauer et al. 2015). The less-popular insurance pollinators, such as flies, beetles, wasps, and butterflies, have gained attention since they provide pollinating services for wild and cultivated plants (Rader et al. 2020). They can compete with bees and other pollinators for floral resources and thus promote or shorten flower visits (Brittain Claire et al. 2013) which in turn might improve pollen transport. Predators might prey on insurance pollinators that can frequently outnumber bees (Kearns and Inouye 1994), and consequently have less impact on the plant’s reproduction and fitness. For both plants and flower visitors, being a generalist has evolutionary advantages (Draper et al. 2021), and they are part of the intricate, multilevel, hypercomplex relationships that occur in nature. With this in mind, we aimed at replicating our study during the flowering season of S. edule in 2020 at a lower altitude (below 1000 m) in the warmer municipality of Ocosingo, Chiapas. It was not possible to gather the same amount of data as for higher elevation, but preliminary data showed an overwhelming majority of visitation to chayote flowers performed by Trigona corvina (Martínez-Bauer unpublished data). This confirms previous reports, based on abundance and efficiency of pollinators at the same altitudes, for Chiapas (CONABIO 2013) and Costa Rica (Heard 1999). We show that other insect genera and orders, apart from stingless bees, are primary pollinators at higher altitudes. Chayote is susceptible to drastic changes in its geographical distribution due to climate change (González-Santos et al. 2017); therefore, a deeper understanding on the ecology of this species might increase the chances to ensure its agricultural, economic, cultural, and pharmaceutical value.
A second highlight of our study is the evidence for a rich and generalized web of interactions between pollinators and plants — including those demeaned as weeds — expressed in the diet breadth of chayote’s pollinators and flower visitors, a trait that varies in relation to resource availability (Fontaine et al. 2008). We demonstrate that A. mellifera can visit up to 48 flowering plants and the native bee B. ephippiatus at least 30, including those with agricultural value and those lacking it, evidencing the need for a rich and varied diet for bees (Vides-Borrell et al. 2019). The same need applies to wasps, such as Vespula squamosa and Agelaia sp. 1, as primary and insurance pollinators of S. edule that also visit a wide spectrum of flowering plants (35 and 24, respectively). The complexity of this pollinator-weed interaction network underlines the importance of plant diversity to sustain the pollination services provided by wild insect populations (Garibaldi et al. 2011). In the case of chayote, grown at altitudes below 2000 m and dependent on Trigona species for fruit production, the yield is frequently diminished because of the use of pesticides (Somá Álvarez and Núñez Grajales 2013). Bees experience sublethal or lethal effects after being exposed to herbicides containing glyphosate (Battisti et al. 2021) or insecticides with neonicotinoids (Lu et al. 2020) or other active ingredients. New and similar agrochemicals are hindering populations of other pollinators (such as wasps) and beneficial predatory insects (Siviter and Muth 2020), affecting the free services insects provide for agroecosystems. Integrated Weed Management systems, other than applying herbicides, are very effective in reducing the undesired effects of weeds (Chikowo et al. 2009), allowing their beneficial ecological services. For example, the association of crops with allelopathic properties controlling the abundance of fast-growing herbs (Bhadoria 2011; Shahzad et al. 2021) or the analysis of the functional value of weed communities in agroecosystems (Bàrberi et al. 2018) are promising techniques. In addition, the planting of non-crop flowering plants that attract pest predators (Tschumi et al. 2015) or pollinators (Carvalheiro et al. 2012) is being increasingly incorporated into land management practices. The closely intertwined features between the natural and economic worlds suggest that above short-term monetary gain, there is a need to prioritize the value of floral resources towards pollination, pest control services, and ultimately food sovereignty.
In the prelude of significant modification in plant distributions due to climate change, the landscapes that provide advantageous conditions or are vegetation refugia (Keppel et al. 2012) will likely allow the persistence of organisms and their ecological services. We add evidence on the importance of various insect orders as pollinators of S. edule at high altitudes, in areas that could become vegetation refugia. In addition, the value of small-scale agroecosystems (Hass et al. 2018) and mixed cropping (Lizarazo et al. 2020) in favor of sustainability (Bonke et al. 2021) is recognized by farmers and scientists alike. Our results contribute to building knowledge on valuing small-scale agroecological production of crops and strengthen the consideration that the control of weeds in crop fields requires a makeover, shifting towards efficient and environmentally smart management proposals (Stokstad 2013).
Data availability
Data available as Online Supplementary Material and upon reasonable request.
Code availability
analyses are standard procedure, but our custom code, if requested, can be provided.
References
Almaguer González JA, García Ramírez HJ, Vargas Vite V, Padilla Mirazo M (2020) Fortalecimiento de la Salud con Comida, Ejercicio y Buen Humor: La Dieta de la Milpa. Modelo de Alimentación Mesoamericana Saludable y Culturalmente Pertinente. Secretaría de Salud, Ciudad de Mexico
Altieri MA, Anderson MK, Merrick LC (1987) Peasant Agriculture and the Conservation of Crop and Wild Plant Resources. Conserv. Biol. 1, 49–58. https://doi.org/10.1111/j.1523-1739.1987.tb00008.x
Arnold N, Ayala R, Mérida J, et al. (2018) Registros nuevos de abejas sin aguijón (Apidae: Meliponini) para los estados de Chiapas y Oaxaca, México. Rev. Mex. Biodivers. 89, 651–665. https://doi.org/10.22201/ib.20078706e.2018.3.2429
Bàrberi P, Bocci G, Carlesi S, et al. (2018) Linking species traits to agroecosystem services: a functional analysis of weed communities. Weed. Res. 58, 76–88. https://doi.org/10.1111/wre.12283
Bates D, Maechler M, Bolker BM (2012) lme4: Linear mixed-effects models using S4 classes. Version 0.999999–0URL. http://CRAN.R-project.org/package=lme4
Battisti L, Potrich M, Sampaio AR, et al. (2021) Is glyphosate toxic to bees? A meta-analytical review. Sci. Total. Environ. 767, 145397. https://doi.org/10.1016/j.scitotenv.2021.145397
Benítez M, Fornoni J, Garcıa-Barrios L, López R (2014) Dynamical networks in agroecology: the milpa as a model system. Separate chapter from the open access book Frontiers in Ecology, Evolution and Complexity. Mexico City. ISBN: 978-1-938128-05-9
Bhadoria PBS (2011) Allelopathy: A Natural Way towards Weed Management. J Exp Agric Int 7–20. https://doi.org/10.9734/AJEA/2011/002
Boege E (2008) El patrimonio biocultural de los pueblos indígenas de México: hacia la conservación in situ de la biodiversidad y agrodiversidad en los territorios indígenas. Instituto Nacional de Antropología e Historia: Comisión Nacional para el Desarrollo de los Pueblos Indígenas, Mexico, D.F
Bonke V, Michels M, Musshoff O (2021) Will Farmers Accept Lower Gross Margins for the Sustainable Cultivation Method of Mixed Cropping? First Insights from Germany. Sustainability 13:1631. https://doi.org/10.3390/su13041631
Brittain C, Williams N, Kremen C, Klein, A-M (2013) Synergistic effects of non-Apis bees and honey bees for pollination services. Proc R Soc B Biol Sci 280:20122767. https://doi.org/10.1098/rspb.2012.2767
Brock RE, Cini A, Sumner S (2021) Ecosystem services provided by aculeate wasps. Biol Rev n/a: https://doi.org/10.1111/brv.12719
Caccavari M and Cilla G (2010) Remoción química como nueva alternativa a la remoción mecánica para el estudio del polen transportado en las escopas de abejas silvestres. Rev. Mus. Argentino Cienc. Nat. n.s. 12(2):255–262–262
Cadena Iñiguez J and Arévalo Galarza Ma de L (2010) GiSeM: rescatando y aprovechando los recursos fitogenéticos de Mesoamérica Volumen 1: Chayote. México. Grupo Interdisciplinario de Investigación en Sechium edule en México, A.C. y Colegio de Postgraduados
Carvalheiro LG, Seymour CL, Nicolson SW, Veldtman R (2012) Creating patches of native flowers facilitates crop pollination in large agricultural fields: mango as a case study. J Appl Ecol 49:1373–1383. https://doi.org/10.1111/j.1365-2664.2012.02217.x
Chikowo R, Faloya V, Petit S, Munier-Jolain NM (2009) Integrated Weed Management systems allow reduced reliance on herbicides and long-term weed control. Agric Ecosyst Environ 132:237–242. https://doi.org/10.1016/j.agee.2009.04.009
Comisión Nacional para el Conocimiento y Us de la Biodiversidad (CONABIO) (2013) La biodiversidad en Chiapas: Estudio de Estado. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad/Gobierno del Estado de Chiapas, Mexico
Draper JT, Haigh T, Atakan O, et al. (2021) Extreme host range in an insular bee supports the super-generalist hypothesis with implications for both weed invasion and crop pollination. Arthropod-Plant Interact 15:13–22. https://doi.org/10.1007/s11829-020-09799-w
Fontaine C, Collin CL, Dajoz I (2008) Generalist foraging of pollinators: diet expansion at high density. J Ecol 96:1002–1010. https://doi.org/10.1111/j.1365-2745.2008.01405.x
Garibaldi LA, Steffan‐Dewenter I, Kremen C, et al. (2011) Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol Lett 14:1062–1072. https://doi.org/10.1111/j.1461-0248.2011.01669.x
González-Santos R, Cadena-Íñiguez J, Morales-Flores FJ, et al. (2017) Prediction of the effects of climate change on Sechium edule (Jacq.) Swartz varietal groups in Mexico. Genet Resour Crop Evol 64:791–804. https://doi.org/10.1007/s10722-016-0401-4
Guevara Hernández F, Rodríguez Larramendi L, Gómez Castro H, et al. (2015) Local criteria for chayote (Sechium edule Jacq. Sw.) seed selection in rural areas of Chiapas, Mexico. Acta Agronómica 64:178–185. https://doi.org/10.15446/acag.v64n2.39776
Gurini LB, Dovico Á, Alvarez AR, Maldonado LM (2020) Producción y procesamiento de polen : buenas prácticas de manejo y manufactura. Ediciones INTA, Buenos Aires, Argentina
Hass AL, Kormann UG, Tscharntke T, et al. (2018) Landscape configurational heterogeneity by small-scale agriculture, not crop diversity, maintains pollinators and plant reproduction in western Europe. Proc R Soc B Biol Sci 285:20172242. https://doi.org/10.1098/rspb.2017.2242
Heard TA (1999) The Role of Stingless Bees in Crop Pollination. Annu Rev Entomol 44:183–206. https://doi.org/10.1146/annurev.ento.44.1.183
INEGI (2020) Prontuario de información geográfica municipal de los Estados Unidos Mexicanos, San Cristóbal de Las Casas, Chiapas
IPBES (2019) Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES secretariat, Bonn, Germany
IPBES (2016) Summary for policymakers of the assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production
Kearns CA, Inouye DW (1994) Fly pollination of Linum lewisii (Linaceae). Am J Bot 81:1091–1095. https://doi.org/10.1002/j.1537-2197.1994.tb15602.x
Kearns CA, Inouye DW (1993) Techniques for Pollination Biologists. University Press of Colorado, Niwot, Colorado, USA
Keppel G, Niel KPV, Wardell-Johnson GW, et al. (2012) Refugia: identifying and understanding safe havens for biodiversity under climate change. Glob Ecol Biogeogr 21:393–404. https://doi.org/10.1111/j.1466-8238.2011.00686.x
Klein A-M, Vaissière BE, Cane JH, et al. (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B Biol Sci 274:303–313. https://doi.org/10.1098/rspb.2006.3721
Lira R, Castrejon J, Zamudio S, Rojas-Zenteno C (1999) Propuesta de ubicación taxonómica para los chayotes silvestres (Sechium edule, Cucurbitaceae) de México. Acta Bot Mex 47–61. https://doi.org/10.21829/abm49.1999.838
Lizarazo CI, Tuulos A, Jokela V, Mäkelä PSA (2020) Sustainable Mixed Cropping Systems for the Boreal-Nemoral Region. Front Sustain Food Syst 0: https://doi.org/10.3389/fsufs.2020.00103
Lu C, Hung Y-T, Cheng Q (2020) A Review of Sub-lethal Neonicotinoid Insecticides Exposure and Effects on Pollinators. Curr Pollut Rep 6:137–151. https://doi.org/10.1007/s40726-020-00142-8
Martínez-Bauer AE, Cerón-Martínez G, Murphy DJ, Burd M (2015) Multitasking in a plant–ant interaction: how does Acacia myrtifolia manage both ants and pollinators? Oecologia 1–11. https://doi.org/10.1007/s00442-014-3215-0
Newstrom LE (1986) Studies in the origin and evolution of chayote, Sechium edule (Jacq.) Sw. (Cucurbitaceae). Doctoral dissertation. University of California, Berkley
Panda P, Appalashetti M, M.A. Judeh Z (2011) Phenylpropanoid Sucrose Esters: Plant-Derived Natural Products as Potential Leads for New Therapeutics. Curr Med Chem 18:3234–3251. https://doi.org/10.2174/092986711796391589
Pimbert MP (2018) Food sovereignty, agroecology and biocultural diversity: constructing and contesting knowledge. Routledge, Abingdon, Oxon, UK
Potts SG, Biesmeijer JC, Kremen C, et al. (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rader R, Bartomeus I, Garibaldi LA, et al. (2015) Non-bee insects are important contributors to global crop pollination. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1517092112
Rader R, Cunningham SA, Howlett BG, Inouye DW (2020) Non-Bee Insects as Visitors and Pollinators of Crops: Biology, Ecology, and Management. Annu Rev Entomol 65:391–407. https://doi.org/10.1146/annurev-ento-011019-025055
Rojas-Sandoval J (2018) Sechium edule (chayote). Invasive Species Compend CABI. https://doi.org/10.1079/ISC.49493.20203482792
Roubik DW, Villanueva Gutiérrez R, Cabrera Cano E, Colli Uca W (1991) Abejas nativas de la reserva de la biósfera de Sian Ka’an, Quintana Roo, Mexico. In: Navarro López D, Robinson JG, Suárez Morales E (eds) Diversidad Biológica en la Reserva de la Biósfera de Sian Ka’an, Quintana Roo, México. Centro de Investigaciones de Quintana Roo, Chetumal, Quintana Roo, Mexico, pp 317–320
Salazar-Aguilar S, Ruiz-Posadas LDM, Cadena-Iñiguez J, et al. (2017) Sechium edule (Jacq.) Swartz, a New Cultivar with Antiproliferative Potential in a Human Cervical Cancer HeLa Cell Line. Nutrients 9:798. https://doi.org/10.3390/nu9080798
Shahzad M, Jabran K, Hussain M, et al. (2021) The impact of different weed management strategies on weed flora of wheat-based cropping systems. PLOS ONE 16:e0247137. https://doi.org/10.1371/journal.pone.0247137
Siviter H and Muth F (2020) Do novel insecticides pose a threat to beneficial insects? Proc R Soc B Biol Sci 287:20201265. https://doi.org/10.1098/rspb.2020.1265
Somá Álvarez LA and Núñez Grajales SF (2013) Estudio socio-agronómico de la producción de chayote (Sechium edule Jac. Swartz), en los municipios de Villaflores y Villa Corzo, Chiapas, México. Universidad Autónoma de Chiapas
Stokstad E (2013) The War Against Weeds Down Under. Science 341:734–736. https://doi.org/10.1126/science.341.6147.734
Tschumi M, Albrecht M, Entling MH, Jacot K (2015) High effectiveness of tailored flower strips in reducing pests and crop plant damage. Proc R Soc B Biol Sci 282:20151369. https://doi.org/10.1098/rspb.2015.1369
Vides-Borrell E, Porter-Bolland L, Ferguson BG, et al. (2019) Polycultures, pastures and monocultures: Effects of land use intensity on wild bee diversity in tropical landscapes of southeastern Mexico. Biol Conserv 236:269–280. https://doi.org/10.1016/j.biocon.2019.04.025
Vieira EF, Pinho O, Ferreira IMPLVO, Delerue-Matos C (2019) Chayote (Sechium edule): A review of nutritional composition, bioactivities and potential applications. Food Chem 275:557–568. https://doi.org/10.1016/j.foodchem.2018.09.146
Warren J (2015) The Nature of Crops: How We Came to Eat the Plants We Do. CABI
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer New York
Wille A, Orozco E, Raabe C (1983) Polinización del chayote Sechium edule (Jacq.) Swartz en Costa Rica. Rev Biol Trop 31:145–154
Yeo HJ, Baek S-A, Sathasivam R, et al. (2021) Metabolomic analysis reveals the interaction of primary and secondary metabolism in white, pale green, and green pak choi (Brassica rapa subsp. chinensis). Appl Biol Chem 64:3. https://doi.org/10.1186/s13765-020-00574-2
Acknowledgements
We are grateful to Karina Tavera for help during fieldwork and to the producers Diega, Jovita, Rosi, Kippy, Domingo, and Angel (all exceptionally committed to agroecological production of food) for kindly granting us access to their parcels and chayote plants. We thank the valuable comments, from David Inouye and an anonymous reviewer, which improved this paper.
Funding
This study was funded by grant 291333 from SADER-CONACYT (Secretaría de Agricultura y Desarrollo Rural – Consejo Nacional de Ciencia y Tecnología), grant 76771 from SEMARNAT-CONACYT (Secretaría de Medio Ambiente y Recursos Naturales—Consejo Nacional de Ciencia y Tecnología) of Mexico, and an Independent Postdoctoral Fellowship from CONACYT (2019–000006-01NACV-00665).
Author information
Authors and Affiliations
Contributions
Angelica Elizabeth Martínez-Bauer conceived this research, designed experiments, and collected data. Gerardo Cerón-Martínez and Rémy Vandame participated in the design. Gerardo Cerón-Martínez performed analyses. Angelica Elizabeth Martínez-Bauer wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Sara Diana Leonhardt
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martínez-Bauer, A.E., Vandame, R. & Cerón-Martínez, G. More than the usual suspect: diversity of pollinators of chayote (Sechium edule) at high elevations in Chiapas, Mexico. Apidologie 52, 1223–1238 (2021). https://doi.org/10.1007/s13592-021-00898-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-021-00898-y