Abstract
Narcissus tazetta L., a bulbous plant belongs to the Amaryllidaceae family, contains alkaloid galantamine (GAL) with acetylcholinesterase inhibitory activity which has been recently considered to treat Alzheimer’s disease (AD). In the current work, the effect of photoperiod (16/8 h light/dark and 24 h dark) and various concentrations of NAA, BAP, and GA3 (0, 0.5, 1 and 2 mg l‒1) on the in vitro mass bulblet regeneration of N. tazetta was studied. The GAL production ability of the regenerated bulblets was assessed by HPLC-UV-MS. Light treatments significantly affected the number of bulblet and leaf, the ratio of bulblet/leaf, and leaf length. The maximum number of bulblet (31.0 ± 1.58) and leaf (13.3 ± 1.33) was recorded from the cultures fortified with NAA and BAP (2 mg l‒1) kept in 16/8 h light/dark, while the maximum leaf length (2.1 ± 0.92 cm) was measured on the MS medium containing 0.5 mg l‒1 NAA and 2 mg l‒1 BAP incubated in the same photoperiod. The average ratio of bulblet proliferation per explant was significantly different between studied photoperiod (1.1 ± 0.86) and 24 h dark (0.62 ± 0.31). The regenerated bulblets contained 40 and 20 µg g‒1 DW GAL underexposed photoperiod and 24 h dark, respectively. This information could be useful in the commercial production of GAL as a valuable anti-AD compound through in vitro mass bulblet proliferation of N. tazetta.
Key message
The regenerated mass bulblets of Narcissus tazetta (Amaryllidaceae) on MS medium containing 2 mg l‒1 NAA and BAP kept in 16/8 h light/dark are recommended to produce galanatamine and lycorine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus of Narcissus L. belonging to the subfamily Amaryllidoideae is one of the fifteen genera of the Amaryllidaceae family, which contains 80 to 100 wild species of perennial bulbiferous geophytes (Berkov et al. 2009; Hanks 2002). The genus is widely distributed in the southwest of Europe, with the Iberian Peninsula diversity center (Spain and Portugal) and North Africa (Ito et al. 1999). Few species of Narcissus have been developed in France and Italy, and some are found in the Balkans and the Eastern Mediterranean regions (Hanks 2002). The genus members are commonly known as daffodil, daffadowndilly, narcissus, and jonquil throughout the world. Narcissus tazetta L. is an indigenous ornamental and fragrant plant species that is distributed from Portugal to Turkey. The plant is also growing from central to eastern Asia (Berkov et al. 2009; Hanks 2002). Indigenous habitats of Narcissus species are diverse and include a range of low-lying lands to mountainous areas as well as lawns, shrubs, forests, and cliffs (Hanks 2002). The genus Narcissus and the other genera of Amaryllidaceae family i.e., Ungernia Bunge, Leucojum L., Galanthus L., Zephyranthes Herb., Hymenocallis Salisb., and Haemanthus L. are well-known alkaloid-containing plants, due to the presence of Amaryllidaceae alkaloids (AAs) including lycorin, crinine, hemanthamine, narciclasine, galantamine, tazettine, homolycorine, montanine, and norbelladine which form an entirely different taxonomic and chemical group (Bastida et al. 2006; Torras-Claveria et al. 2013).
Galantamine (GAL) is a well-known long-acting, reversible AAs, and a competitive inhibitor for the acetylcholinesterase (AChE) enzyme and a nicotine receptor allosteric modifier for acetylcholine (Maelicke 2000; Woodruff-Pak et al. 2002). GAL can cross from the blood-brain barrier and effect on the central nervous system (Bastida et al. 2006; Ghosal et al. 1990). Due to the ability of GAL to enhance central cholinergic function, it has been introduced in Europe to treat Alzheimer’s disease (AD) since 1980 (Bores and Kosley 1996). GAL-hydrobromide as a superior pharmaceutical profile has a higher tolerance to the major AChE inhibitors, including physostigmine or tacrine, and is currently used in the United States under the name Razadine®, known as Reminyl®, to treat certain stages of AD (Grutzendler and Morris 2001; Heinrich and Teoh 2004). Pharmaceutical demand of GAL has dramatically increased due to its significant effect on memory enhancement and AD control. The cost of GAL production is determined based on the quality of plant raw materials and their alkaloid content. The phytochemical characteristics of the plant materials were also affected by the extraction efficiency, which finally led to increase the costs of GAL production. Bulbs of Narcissus species and the other members of the Amaryllidaceae family including Leucojum aestivum L., Ungernia victoris Vved. ex Artjush., and Lycorice radiata Herb. have been reported as GAL-rich natural sources (Abou‐Donia et al. 2008; Berkov et al. 2009).
Different phytochemical patterns of plants due to the environmental changes, low content of their secondary metabolites (SMs), and a limitation for their materials collection from the natural habitats show the need to pay attention to the production of SMs by biotechnological methods (Ravishankar and Ramachandra Rao 2000). In the past decades, in vitro culture techniques have been used to produce massive amounts of many SMs in a short time, especially for types with valuable pharmaceutical effects (Malik et al. 2011). Biotechnology, through in vitro plant cell, tissue, and organ culture (PCTOC) enables the production of plant bioactive compounds under stable and controlled conditions in a shorter period. Mainly, PCTOC has been assessed in the study of plant developmental processes, physiological changes, production, and extraction of SMs. Therefore, these techniques can be simultaneously used for mass propagation, cloning, and the production of SMs from cultured organs or cells to provide the high demand for pharmaceutical industries (Bonfill et al. 2013; Malik et al. 2011; Sarmadi et al. 2019).
In a variety of plant species, especially monocotyledons, callus induction and establishment of cell culture are difficult, so the multiplication of their medicinal organs through in vitro cultures can be alternatively used to produce a remarkable amount of target bioactive compounds. Micropropagation of various bulbous plants has been so far reported (Haque and Ghosh 2016; Juan-Vicedo et al. 2019; Trujillo-Chacón et al. 2020; Ulrich et al. 1999; Zayed et al. 2011). In vitro bulb regeneration, callus induction, and somatic embryogenesis in several species and varieties of Narcissus have also been reported (Bergoñón et al. 1996; Chow et al. 1992; Colque et al. 2004; Abdel-Rahman et al. 2017; Malik and Bach 2017; Sage et al. 2000; Santos and Salema 2000; Santos et al. 1998; Sellés et al. 1997; Sochacki 2008; Staikidou et al. 2005).
Our literature survey revealed that in vitro propagation of N. tazetta and its varieties has already been reported (Chen et al. 2005; Chen and Ziv 2005; Zhang et al. 2013; Abdel-Rahman et al. 2017). To the best of our knowledge, the effect of plant growth regulators (PGRs), photoperiod and their interaction on the in vitro bulblet proliferation of the plant has not been studied yet. In the following of our recent work on the quantification of GAL in the Iranian populations of N. tazetta (Rahimi Khonakdari et al. 2018), the present study aimed to gain insight into the effect of photoperiod and various types and concentration of PGRs on bulb multiplication and biomass production of the plant in culture condition in vitro. This information can be used in the semi-industrial production of the important anti-AD compound GAL and the other AAs through controlled N. tazetta bulblet culture.
Materials and methods
Plant material
Healthy bulbs were collected from N. tazetta cultivated in Matan Kola village, Aliabad Rural, Ghaemshahr, Mazandaran Province in November 2018 and used as explants source (Fig. 1A–1C). Geographical and edaphological characteristics of the collection site are presented in Table 1.
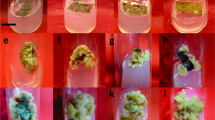
In vitro bulblet proliferation of Narcissus tazetta L. Map of collection site of the plant material studied (A), The plant used as explants source (B), The fresh and healthy bulbs (C), washed and sterile bulb used for explants excision (D), Sterile two-scale explants (E), established culture (F), proliferated bulblets on the MS medium containing NAA and BAP (2 mg l‒1) kept in 16/8 h light/dark (G)
Sterilization and culture establishment
The plant bulbs were cleaned and washed carefully. Outer and dried scales were then removed and the rest bulbs (Fig. 1D) were washed under running tap water for 48–72 h. The bulbs were then incubated for 1 h in a bath of water at 49 °C to eliminate endophytes. The bulbs were then floated in sterile distilled water containing a few drops of Tween 80 for an hour. In the next step, the bulbs were immersed in 70% ethanol for 2 min and then washed with sterile distilled water for 2 min. The bulbs were then immersed in a 2% NaOCl solution containing a few drops of Twin 20 for 25 min with slow shaking. The bulbs were finally washed three times with sterile distilled water at intervals of 2, 5, and 7 min, and placed on filter paper. Decontaminated bulbs are divided into two-scale explants (Fig. 1E) which comprise of two adjacent scales connected by a piece of basal plate tissue (1 cm²) and were then placed on the MS medium containing 30 g l‒1 sugar and 8 g l‒1 agar supplemented with different concentrations of PGRs (Fig. 1F).
In vitrobulb proliferation.
Sterile two-scale explants of N. tazetta were transferred to the baby food jars containing prepared MS media (40 ml) containing different concentrations (0, 0.5, 1, and 2 mg l‒1) of BAP, NAA, and GA3 (Table 2). MS medium without PGRs was also considered as a control. The induction of bulblet was also studied under two light regimes including 16/8 h light/dark provided by cool-white fluorescent lamps at a photon flux density of 40 µmol m− 2 s− 1 and 24 h dark kept in a paper box. All cultures were incubated in a growth chamber at 25 ± 2 °C. Each baby food jar was considered as an experimental unit, and the experiment was done in triplicates per treatment. In total, five jars were considered as experimental units in each replicate. Four two-scale explants were cultured in each jar. The number of bulblet and leaf, the ratio of bulblet/leaf, and leaf length were measured after four weeks (Fig. 1G).
Extraction and HPLC-DAD-MS analysis
Four-week-old proliferated bulblets comprising photosynthetically active leaves (1–2 cm) were used for the extraction of alkaloids. Extraction, high-performance liquid chromatography (HPLC)-photo-diode array detector (DAD), and HPLC-mass spectrometry (MS) analyses have been conducted as described previously (Georgieva et al. 2007; Rahimi Khonakdari et al. 2018). For instance, 15 milliliters of methanol were added to 300 mg of completely powdered freeze-dried bulblets and then sonicated for 15 min at room temperature. This stage was repeated thrice. The mixture was then centrifuged for 15 min at 4400 rpm and passed through the filter. The extracts were dried using a rotary evaporator (Heidolph Instruments GmbH, Schwabach Germany). The dried extract was re-dissolved in 4 ml H2SO4 3% and defatted with 5 ml diethyl ether (n = 03). After basification to pH 9–10 with 25% ammonia, the GAL was extracted with chloroform (3 × 5 ml). The organic solvent was dried under reduced pressure in a rotary evaporator at 40 °C. The extracts were then dissolved in HPLC grade methanol (1 ml), filtered through a Millipore filter (0.45 mm) and used for analysis.
Galantamine quantification proceeded on a KNAUER liquid chromatography apparatus consisting of a 1000 Smartline Pump, a 5000 Smartline Manager Solvent Organizer and a 2800 Smartline Photo-DAD was used for the HPLC analysis. The injection was carried out through a 3900 Smartline Autosampler injector equipped with a 100 µl loop. The temperature of the column was controlled with a Jet Stream 2 Plus oven (Knauer, advanced scientific instrument, Berlin, Germany). The separation was achieved on a 25 cm × 4.6 mm with a pre-column, Eurospher 100-5 C18 analytical column provided by Knauer (Berlin, Germany). Data acquisition and integration was performed with EZchrom Elite software. The elution was carried out in a gradient system with acetonitrile as the organic phase (solvent A) and 1% (w/v) ammonium acetate buffer adjusted to pH 6.6 with acetic acid (solvent B) with the flow-rate of 1 ml min− 1. Peaks were monitored at 280 nm wavelength. Injection volume was 20 µl and the temperature was maintained at 25 °C. All injections were repeated three times (n = 03). Calibration graphs were plotted subsequently for linear regression analysis of the peak area with concentration 5, 10, 25, 50, 80, 120, 150, and 200 mg l− 1. The standard of GAL was purchased from Sigma (Sigma-Aldrich Corporation, MO, USA). Methanol, HPLC grade methanol, acetonitrile, and ammonium acetate of analytical grade were obtained from Merck (Darmstadt, Germany). HPLC grade water was used throughout the analysis.
HPLC-MS separations were carried out on an Agilent series 1200 system equipped with degasser, binary high pressure mixing pump, column thermostat, and variable-wavelength UV-Vis absorbance detector (Agilent Technologies; Waldbronn, Germany). Using the same chromatographic condition, all eluted peaks were transferred to a Finnigan TM LCQ TM DECA instrument, comprising an ion trap. An ionization device was used for sample analyses (sheath gas: 80 ml/min; auxiliary gas: 20 ml min; spray voltage: 5 kV; capillary temperature: 300 °C; capillary voltage: 46 kV; tube lens: ‒ 60 kV). The Xcalibur 2.0 SR2 software (copyright Thermo Electron Corporation 1998–2006) was used.
Statistical analysis
The experiment was a factorial design in a completely randomized design (CRD) with three replications. Data analysis was performed using SAS statistical software version 1.3.1. The means were compared using the Duncan multi-domain test at the probability level of 0.05, and according to their results, the charts were plotted using Excel software. The following equations calculate the ratio of bulb production and shoot formation indices:
Bulblet formation ratio index = number of explants/number of produced bulblets.
Shoot formation ratio index = number of explants/number of produced shoots.
Results
In the present study, the used sterilization protocol as mentioned in the experimental comprising 70% ethanol, 2% NaOCl solution, and a few drops of Twin 20 showed satisfactory results on the control of surface contamination of the cultured two-scale explants. Pre-treatment of the explants with hot water before surface sterilization with sodium hypochlorite was additionally used. The explants incubation in hot water (49 °C) for an hour gave the best result for the disinfection of endophytes contamination of N. tazetta bulbs.
The results of the variance analysis revealed that hormonal treatments, light conditions, and their interaction had significant effects on the number of bulblet and leaf, the ratio of bulblet/leaf, and leaf length of N. tazetta (Table 3). The results showed that there was a significant difference (P < 0.05) between treatments of photoperiod (16/8 h light/dark) and 24 h dark on the number of bulblet and leaf, and the ratio of each of them, as well as leaf length, so that the maximum number of bulblet (13.5 ± 1.44) and number of leaf (6.2 ± 0.87) were obtained under 16/8 h light/dark. It can be concluded that photoperiod is necessary for leaf production. The effect of light on the leaf length was also significant, so that, in light and dark conditions, the produced leaves were 0.86 ± 0.46 and 0.26 ± 0.22 cm, respectively. The mean ratio of bulblet production per sample was also significantly different between dark and photoperiod conditions. The mean value for light and dark conditions was 1.1 ± 0.86 and 0.62 ± 0.31 bulblet/explant, respectively.
In the study of the interaction effect of PGRs and light conditions, the highest number of bulblet (31.0 ± 1.6) and leaf (13.3 ± 1.3) were observed in the two-scale explants cultured on MS medium containing 2 mg l‒1 of both BAP and NAA (treatment no. 9) under photoperiod condition (Fig. 2), but the highest leaf length (2.1 ± 0.52 cm) was observed on the treated explants with 2 mg l‒1 BAP and 0.5 mg l‒1 NAA (treatment no. 3) under light condition. The highest number of bulblet (28.6 ± 1.2) in continuous dark (24 h dark) was obtained from treatment no. 2 (0.5 mg l‒1 NAA and 1 mg l‒1 BA). The number of the produced leaf under 24 h dark was much less than the exposed photoperiod so that the highest number of the leaf (2.6 ± 0.92) was measured in treatments no. 2, 6, and 7 under continuous dark (Fig. 2). No bulblet was produced on the hormone-free medium in both photoperiod (16/8 h light/dark) and 24 h dark conditions.
The effect of photoperiod and plant growth regulators on the average number of bulblet (A) and shoot (B) proliferated from two-scale explants of Narcissus tazetta. Different letters showed significant differences among treatments at the level (P < 0.05). For a detailed description of treatment code cf. Table 2
The average number of proliferated bulblet among all studied treatments in the light condition (16/8 h light/dark) was approximately twice than 24 h dark conditions. Among treatments containing GA3, treatment no. 13 (1 mg l‒1 GA3, 0.5 mg l‒1 BAP, and 0.5 mg l‒1 NAA) produced the highest number of the leaf (5.3 ± 0.86).
The average ratio of bulblet production per explant under 24 h dark and light conditions with different hormonal treatments is demonstrated in Fig. 3. In exposed photoperiod (16/8 h light/dark) and 24 h dark, the highest ratio of bulblet production was observed in treatments nos. 9 and 2, respectively. As can be seen, the highest percentage of leaf production ratio per explant (1.1) was in hormonal treatment no. 9 under 16/8 h light/dark, while the ratio in the treatments nos. 2, 6, and 7 under 24 h dark was up to 0.26 leaf per explant. The maximum mean of shoot length (2.1 ± 0.76 cm) was obtained from treatment no. 3 underexposed photoperiod, while this treatment did not produce leaf in 24 h dark. The highest leaf length (0.83 ± 0.42 cm) in the dark condition was obtained from treatment no. 2. Treatment no. 9 showed a significant difference with other tested treatments and produced the highest leaf ratio in each explant. Among studied treatments containing GA3, the maximum leaf length (0.86 ± 0.38 cm) was obtained in treatment no. 10 (Fig. 3).
The effect of photoperiod and plant growth regulators on the ratio of bulblet (A) and shoot (B) proliferated from two-scale explants of Narcissus tazetta. Different letters showed significant differences among treatments at the level (P < 0.05). For a detailed description of treatment code cf. Table 2
HPLC-UV-MS profiling of the methanolic extract of the regenerated bulblets of N. tazetta is shown in Fig. 4. Characteristically, the standard GAL (C17H21NO3) yielded an [M+H]+ peak at a molecular weight of 288.6 Da (the corresponding exact mass for GA is 288.1594 Da). The peaks corresponding to GAL exhibited mass-to-charge (m/z) ratios corresponding to the molecular ions [M+H]+ of authentic GAL (at 9.77 min), confirming the presence of GAL in the methanolic extract of the proliferated bulblets. Extracted ion chromatogram for 288.1594 Da (the exact mass related to GAL) led to find an isobaric compound of GAL. According to the LC-MS data, the presence of lycorine (C16H17NO4) in the methanolic extract of the studied in vitro proliferated bulblets was also confirmed. The exact mass for this compound and its corresponding retention time are 288.1230 Da and 5.6 min, respectively (Fig. 4). Quantitative HPLC determination showed that the regenerated bulblets on the best proliferation treatment underexposed photoperiod and 24 h dark produced 40 and 20 µg g‒1 DW GAL, respectively, while GAL has not been detected in the produced leaf.
HPLC-UV-MS profiling of the methanolic extract of the regenerated bulblets of Narcissus tazetta L. HPLC-UV chromatogram recorded at 288 nm (A), corresponding total ion current chromatogram (TIC) (B), mass spectrum ([M+H]+) related to the of lycorine (C), and and mass spectrum ([M+H]+) related to galantamine (D)
Discussion
The greatest problem in the in vitro culture techniques such as micropropagation, somatic embryogenesis, embryo rescue, somatic hybridization, somaclonal variation, and PCTOC for the production of SMs is the contamination. A wide range of microorganisms, including fungi, yeasts, bacteria, and viruses are the main contributing factors in the contamination of in vitro cultures mentioned above (Langens-Gerrits and De Klerk 1999). Therefore, sterilization of the plant tissues along with other materials used in the in vitro techniques is one of the most basic and the most important step in the establishment of an in vitro culture.
The main challenge in this regard is the disinfection of explants because the intrinsic contaminants of plant tissues (endophytes) cannot be eliminated using common surface disinfectants and methods. Although this problem cannot be observed in meristem culture, the use of an effective method for the disinfection of the other materials, especially underground explants including bulbs, corms, tubers, and rhizomes is very necessary. Similar to the other bulbiferous plants such as Lilium and Tulips, bulbs of Narcissus contain a wide range of endophytes which can be contaminated the plant in vitro cultures.
In this study, the results of the disinfection and establishment of explants showed that sodium hypochlorite even at high concentrations and longtime did not affect the elimination of endophytes contamination. Although the use of mercuric chloride (0.1%) has been reported for the explant sterilization of some bulbous plants as Zephyranthes grandiflora (Gangopadhyay et al. 2010), and Lilium polyphyllum D. Don ex Royle (Taha et al. 2018), but it has not been considered in the present study due to the toxicity and health risk of its using. Abdel-Rahman et al. (2017) observed the best disinfection results for the bulbs of N. tazetta “Chinensis” through soaking the bulbs in 70% ethanol for 10 min followed by soaking bulbs in 2% HgCl2 for 10 min. The bulb disinfection of Leucojum with 70% ethanol (1 min) and domestos bleach (5% chlorine) 50% and 30% at intervals of 30 and 10 min has been reported (Stanilova et al. 2010). Treatments of the explants with hot water (40–50 °C for 1 to 4 h) followed by drying before sterilizing them with hypochlorite solutions have also been suggested (Teixeira da Silva et al. 2016). The use of hot water at 40 and 54 °C has been reported to eliminate the internal contamination of Lilium and Narcissus bulbous explants, respectively (Langens-Gerrits et al. 1998; Lagnes-Gerrits and De Klerk 1999).
In vitro organogenesis can be affected by several factors such as plant genotype, storage conditions, explant type, polar orientation of the explants, photoperiod, temperature, composition of culture medium, and PGRs ( Chen and Ziv 2005; Abdel-Rahman et al. 2017; Begum et al. 2019; Cheesman et al. 2010; García-Fortea et al. 2020; Nasircilar et al. 2011; Sairam et al. 2003). Photoperiod and concentration of PGRs are probably the most important influencing factors on in vitro organogenesis in the bulbous plants. In vitro propagation of Amaryllidaceae members through scale explants comprising a piece of basal plate has also been reported (Ault 1995; Diop et al. 2006; Fennell et al. 2004; Rice et al. 2011). It has been claimed that adventitious shoots were successfully proliferated from tissue at the base of bulb scales in many bulbous species (Ozel et al. 2015; Rice et al. 2011). So, it is necessary to include the basal plate as part of the scale explant for further bulblet proliferation. Ozel et al. (2007) reported that the combination of BAP-NAA has a significant effect on the proliferation of Muscaria bulbs from two-scale explants, which is consistent with the results obtained in this study (Ozel et al. 2007). An efficient in vitro regeneration of N. tazetta “Chinensis” through twin-scale explants with basal plate cultured on MS medium supplemented with 1 mg l‒1IBA + 1 mg l‒1 triiodobenzoic acid (TIBA) and activated charcoal has been reported (Abdel-Rahman et al. 2017). The highest proliferation bulbs of Muscari mirum Speta has also been reported from four-scale explants cultured on MS medium containing 4 mg l‒1 BAP and 0.25 mg l‒1 NAA (Nasircilar et al. 2011). Rice et al. (2011) reported the highest bulblet induction from twin-scale explants of Brunsvigia undulata F.M. Leight cultured on hormone-free medium. In some reports, both increasing and decreasing effects of TDZ concentration on bulblet induction of bulbous plants have been reported (Huetteman and Preece 1993; Thomas 2007), which has an increasing effect when used in combination with NAA. So, the synergistic effect of NAA in combination with cytokinins has been claimed in the bulblet formation of bulbous plants.
The significant effects of previous bulb storage on the performance of twin-scales for in vitro regeneration of N. tazetta have been reported by Chen and Ziv (2005). They revealed that the twin-scale explants isolated from the bulbs after three months in storage at 30 °C and a six week cold treatment at 15 °C produced high numbers of uniform adventitious buds. In their results, the elongated buds regenerated from inflorescence stem discs had a wide range of length variation when compared to adventitious shoots regenerated from twin-scale explants. They have also been studied the effect of polar orientation on regeneration of the inflorescence stem of N. tazetta which may be due to the presence of growth substances, especially the ratio of cytokinin and auxin within the explants. It has been claimed that the interactions between growth substances and the polar orientation of the explants, and their effects on the regeneration can be a complex phenomenon (Chen and Ziv 2005).
The bulblet induction on the PGRs-free medium has also been reported in many other Amaryllidaceae members including Amaryllis belladonna L. (De Bruyn et al. 1992), Pancratium maritium L. (Dragassaki et al. 2001), Crinum macowanii Baker. (Slabbert et al. 1993), and B. undulata (Rice et al. 2011). In this case, it can be concluded that the explant contains a high level of endogenous PGRs to induce bulblet formation. Although the effect of the genotype and the size of the explant on the bulb regeneration is confirmed, but the effect of NAA-cytokinin composition on the efficient production of bulbs in some other bulbous plants such as Iris sanguinea (Wang et al. 2018), Lachenalia montana (Aremu et al. 2015), Fritillaria ruthenica Wikstr. (Muraseva and Novikova 2018), Muscari muscarimi (Ozel et al. 2015), and L. aestivum (Ptak et al. 2013) have also been reported. The increasing effect of paclobutrazol and sucrose levels on the bulblet induction of L. aestivum in culture condition in vitro has been reported (Ptak et al. 2013). Sultana et al. (2010) studied the effect of BAP and chlorocholine chloride (CCC) concentrations in addition different levels of sucrose on the bulb proliferation of Hippeastrum hybridum and showed that the maximum bulb production was obtained on the medium culture supplemented with 6 mg l‒1 BAP, 500 mg l‒1 CCC, and 90 g l‒1 sucrose (Sutlana et al. 2010). The increasing effect of polyamines and methyl jasmonate on the bulb formation of Tulipa has been reported by Podwyszynska et al. (2015).
The effect of photoperiod on the regeneration of bulbs in A. belladonna (De Bruyn et al. 1992), Lilium longiflorum (Niimi and Onozawa 1979), B. undulate (Rice et al. 2011), and Eucomis zambesiaca (Cheesman et al. 2010) has also been reported. The growth and developmental process of plants in in vitro cultures are significantly affected by the quality of light (color and wavelength), intensity, and photoperiod. Light is also often a key factor in the accumulation of SMs (Yang et al. 2018). The effect of different photoperiods on the regeneration rate of Paulownia under in vitro culture conditions has been reported (Yang et al. 2013). They approved that different light conditions can be affected by the leaf and root induction of the plant. In the contrary, Houllou et al. (2015) showed that photoperiod did not effect on shoot and root development of Capraria biflora L. while affected on the plant callus induction. The effect of photoperiod (16/8 h light/dark) on the bulb production from two-scale explants of B. undulata in in vitro condition has been reported (Rice et al. 2011). Cheesman et al. (2010) showed that 24 h dark completely inhibited bulblet formation in Eucomis zambesiaca. They have been found that the best bulblet induction occurred in the 8 h light cycle. Conversely, the highest bulblet regeneration in Hyacinthus orientalis (Kim et al. 1981) and L. longiflorum (Kumar et al. 2006) has been reported in continuous darkness which is in the opposite of our obtained results. The difference in results observed for N. tazetta could be due to the plant genotype and the type of explant used. Further studies on light conditions (quality and intensity) on the plant need to be investigated to optimize the real effect of light.
It has been revealed that environmental parameters such as light and day length interact with PGRs to cause developmental responses in plants (Cheesman et al. 2010; Kumar et al. 2006; Rice et al. 2011) which are in agreement with obtained results in the present study. Notably, the bulblet production decreased in the treatments containing GA3 (No. 10-18). Thus, it can be concluded that this plant hormone has a negative effect on bulb production in N. tazetta. This is in agreement with obtained results by Pierik and Steegmans (1975) in the bulb proliferation of hyacinth and E. zambesiaca (Pierik and Steegmans 1975).
Generally, it can be concluded that low concentration of auxin and cytokinin is more suitable and efficient for bulb production, while increasing concentrations of these hormones, especially cytokinins, increased the production of the leaf. Santos et al. (2002) reported similar results in in vitro culture of N. bulbocodium L. using MS medium with two different hormonal compositions (BAP 4 mg l‒1 + NAA 12 mg l‒1 and BAP 2 mg l‒1 + IBA 1 mg l‒1) which resulted in leaf induction on explants. In the second hormonal composition, a few small bulblets appeared after 70 days. Growth of proliferated bulblet was carried out with two subcultures on the same culture medium with an increase of 9% sucrose and with or without NAA. They revealed that NAA has not a significant effect on the bulblet size, but led to a better formation of the root. In in vitro propagation of Narcissus, the effect of BAP and NAA on the leaf induction has also been reported by Hussey (1982).
Bergonon et al. (1996) showed that GAL production was stimulated by prolonged photoperiod in the shoots culture of N. confuses which has been reached to 2.5 mg g‒1 DW in the plant tissue culture Berkov et al. (2009) also claimed that light is an important factor in the biosynthesis of GAL in the shoots of L. aestivum cultured in vitro. They found that GAL production in the light conditions is nearly twice that of dark conditions, which is in agreement with obtained results in the present study. The concentration of GAL in different plant organs of L. aestivum has been reported between 0.02 and 0.60 mg g‒1 DW. As the other monocotyledons, callus induction and cell suspension culture establishment in Narcissus is relatively difficult. So, optimization of mass bulb proliferation of the plant can be considered as an efficient and alternative method for the production of medicinally important compound GAL. So far, several attempts for the production of GAL through in vitro cultures of the Amaryllidaceae family have been done. The effect of elicitation on the production of GAL in the shoot culture of N. confuses has been reported (Colque et al. 2004). Quantification of GAL in bulbs and in vitro cultures of N. papyraceus and four varieties of N. tazetta grown in Iran using GC-MS analysis has been recently reported (Tarakemeh et al. 2019). It has been shown that the content of GAL ranged from 6 to 105 µg g‒1 DW among the studied species and varieties. They also showed that GAL accumulation was significantly stimulated by 4′-O-methylnorbelladine precursor in in vitro culture samples of N. tazetta which has been reached to 1.7 and 82 µg g‒1 DW in the calli and bulblet cultures, respectively. Production of GAL through in vitro tissue culture of N. pseudonarcissus cv. Carlton has been currently reported by Ferdausi et al. (2020). According to the obtained LC-MS results comparing with the previous report (Karakoyun and Unver-Somer 2019), lycorine, an Amaryllidaceae alkaloid, was also found in the proliferated N. tazetta bulblets. Interestingly, lycorine is a well-known natural alkaloid with a wide range of pharmaceutical properties (Roy et al. 2018). Antiviral activity of lycorine against severe acute respiratory syndrome-associated coronavirus (SARS-CoV), measles virus, coxsackie virus, poliovirus, human immunodeficiency virus (HIV-1), and herpes simplex virus type 1 has been reported (Szlávik et al. 2004; Li et al. 2005). Therefore, further studies including optimization of medium culture conditions, elicitation, precursor feeding, and the other enhancement strategies can be recommended to produce this valuable alkaloid through developed in vitro mass bulblets culture of N. tazetta.
Conclusions
The chemical synthesis of GAL has been successfully reported, but the main natural source for its commercial production is the plant materials of Amaryllidaceae members. The biomass production rate in these plants is very slow and time-consuming, so in vitro culture establishment of the plants like N. tazetta can be a good way to achieve maximum production of this medicinally important compound for further pharmaceutical purposes and commercial exploitation. Since GAL accumulation in the bulbs of Narcissus is higher than in the shoots, therefore, the present study focused on in vitro mass bulb production of the plant. This study showed that two-scale bulb explants of N. tazetta cultured on the MS medium supplemented with 2 mg l‒1 of both BAP and NAA under photoperiod condition produced the highest number of bulblet. Media culture containing GA3 decreased bulblet proliferation in N. tazetta. It is worthy to note that more bulblets obtained from the medium supplemented with lower hormonal concentrations, which can be considered to reduce production costs in the mass bulblet culture of N. tazetta in the bioreactor for the commercial production of anti-AD compound GAL and medicinally important antiviral component lycorine as well.
Abbreviations
- GAL:
-
Galantamine
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- SMs:
-
Secondary metabolites
- PCTOC:
-
Plant cell, tissue, and organ culture
- PGRs:
-
Plant growth regulators
- AAs:
-
Amaryllidaceae alkaloids
- MS:
-
Murashige and Skoog
- BAP:
-
6-Benzylaminopurine
- NAA:
-
1-Naphthalene-acetic acid
- HPLC:
-
High-performance liquid chromatography
- LC-MS:
-
Liquid chromatography-mass spectrometry
- CRD:
-
Completely randomized design
- TDZ:
-
Thidiazuron
- FW:
-
Fresh weight
- DW:
-
Dry weight
- TIBA:
-
Triiodobenzoic acid
- CCC:
-
Chlorocholine chloride
References
Abdel-Rahman H, Al-Ansary AMF, Rashed KN, Rizkalla AA (2017) Micropropagation of Narcissus tazetta ‘Chinensis’ and its relation to secondary metabolites content. J App Life Sci Int 14(1):1–11
Abou-Donia AH, Toaima SM, Hammoda HM, Shawky E (2008) New rapid validated HPTLC method for the determination of galanthamine in Amaryllidaceae plant extracts. Phytochem Anal 19(4):353–358
Aremu A, Dole¿al K, Van Staden J (2015) New cytokinin-like compounds as a tool to improve rooting and establishment of micropropagated plantlets. In: VI international symposium on production and establishment of micropropagated plants, vol 1155, pp 497–504
Ault JR (1995) In vitro propagation of Eucomis autumnalis, E. comosa, and E. zambesiaca by twin-scaling. HortScience 30(7):1441–1442
Bastida J, Lavilla R, Viladomat F (2006) Chemical and biological aspects of Narcissus alkaloids. In: Cordel GA (ed) The alkaloids: chemistry and biology. Elsevier Inc, Amsterdam, pp 87–179
Begum N, Zenat EA, Sarkar MK, Roy K, Munshi C, Jahan JL, Miskat A (2019) Micro propagation of soybean (Glycine max L.) BARI-5 variety. Open Microbiol J 13(1):177–187
Bergoñón S, Codina C, Bastida J, Viladomat F, Melé E (1996) Galanthamine production in “shoot-clump” cultures of Narcissus confusus in liquid-shake medium. Plant Cell Tiss Org Cult 45(3):191–199
Berkov S, Georgieva L, Kondakova V, Atanassov A, Viladomat F, Bastida J, Codina C (2009) Plant sources of galanthamine: phytochemical and biotechnological aspects. Biotech Biotechnol Equip 23(2):1170–1176
Bonfill M, Malik S, Mirjalili MH, Goleniowski M, Cusido R, Palazon J (2013) Production and genetic engineering of terpenoids production in plant cell and organ cultures. In: Merillon KG (ed) Natural products. Ramawat, Berlin, pp 2761–2796
Bores G, Kosley R (1996) Galanthamine derivatives for the treatment of Alzheimer’s disease. Drugs Future 21(6):621–635
Cheesman L, Finnie J, Van Staden J (2010) Eucomis zambesiaca Baker: factors affecting in vitro bulblet induction. S Afr J Bot 76(3):543–549
Chen J, Ziv M (2005) The effects of storage condition on starch metabolism and regeneration potentials of twin-scales and inflorescence stem explants of Narcissus tazetta. In Vitro Cell Dev Biol—Plant 41:816–821
Chen L, Zhu X, Gu L, Wu J (2005) Efficient callus induction and plant regeneration from anther of Chinese narcissus (Narcissus tazetta L. var. chinensis Roem). Plant Cell Rep 24(7):401–407
Chow Y, Selby C, Harvey B (1992) A simple method for maintaining high multiplication of Narcissus shoot cultures in vitro. Plant Cell Tiss Org Cult 30(3):227–230
Colque R, Viladomat F, Bastida J, Codina C (2004) Improved production of galanthamine and related alkaloids by methyl jasmonate in Narcissus confusus shoot-clumps. Planta Med 70(12):1180–1188
De Bruyn M, Ferreira D, Slabbert M, Pretorius J (1992) In vitro propagation of Amaryllis belladonna. Plant Cell Tiss Org Cult 31(3):179–184
Diop MF, Ptak A, Chrétien F, Henry M, Chapleur Y, Laurain-Mattar D (2006) Galanthamine content of bulbs and in vitro cultures of Leucojum aestivum L. Nat Prod Commun 1(6):475–479
Dragassaki M, Economou A, Vlahos J (2001) Bulblet formation in vitro and plantlet survival extra vitrum in Pancratium maritimum L. In: I International symposium on acclimatization and establishment of micropropagated plants, vol 616, pp 347–352
Fennell C, Van Staden J, Bornman C (2004) Biotechnology of southern African bulbs. S Afr J Bot 70(1):37–46
Ferdausi A, Chang X, Hall A, Jones M (2020) Galanthamine production in tissue culture and metabolomic study on Amaryllidaceae alkaloids in Narcissus pseudonarcissus cv. Carlton Ind Crops Prod 144:112058
Gangopadhyay M, Chakraborty D, Dewanjee S, Bhattacharya S (2010) Clonal propagation of Zephyranthes grandiflora using bulbs as explants. Biol Plant 54(4):793–797
García-Fortea E, Lluch-Ruiz A, Pineda-Chaza BJ, García-Pérez A, Bracho-Gil JP, Plazas M, Gramazio P, Vilanova S, Moreno V, Prohens J (2020) A highly efficient organogenesis protocol based on zeatin riboside for in vitro regeneration of eggplant. BMC Plant Biol 20(1):6
Georgieva L, Berkov S, Kondakova V, Bastida J, Viladomat F, Atanassov A, Codina C (2007) Alkaloid variability in Leucojum aestivum from wild populations. Z Naturforsch C 62:627–635
Ghosal S, Singh SK, Unnikrishnan SG (1990) Effects of stress on alkaloid metabolism in Crinum asiaticum. Phytochemistry 29(3):805–811
Grutzendler J, Morris JC (2001) Cholinesterase inhibitors for Alzheimer’s disease. Drugs 61(1):41–52
Hanks GR (2002) Narcissus and daffodil: the genus Narcissus. CRC Press, Boca Raton
Haque SM, Ghosh B (2016) High-frequency somatic embryogenesis and artificial seeds for mass production of true-to-type plants in Ledebouria revoluta: an important cardioprotective plant. Plant Cell Tiss Org Cult 127(1):71–83
Heinrich M, Teoh HL (2004) Galanthamine from snowdrop—the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J Ethnopharmacol 92:147–162
Houllou LM, de Santana ERB, de Andrade Lima CS, de Souza RA, de Castro Torres GR, Alves GD, Yara R (2015) Effect of light, explant maturity and antioxidant on in vitro organogenesis and plant regeneration of the medicinal plant Capraria biflora L. Res Biotechnol 6(3):24–30
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss Org Cult 33(2):105–119
Hussey G (1982) In vitro propagation of Narcissus. Ann Bot 49(5):707–719
Ito M, Kawamoto A, Kita Y, Yukawa T, Kurita S (1999) Phylogenetic relationships of Amaryllidaceae based on matK sequence data. J Plant Res 112(2):207–216
Juan-Vicedo J, Pavlov A, Ríos S, Casas JL (2019) In vitro culture and micropropagation of the Baetic-Moroccan endemic plant Lapiedra martinezii Lag. (Amaryllidaceae). In Vitro Cell Dev Biol Plant 55(6):725–732
Karakoyun C, Unver-Somer N (2019) Simultaneous quantitative analysis of biologically important Amaryllidaceae alkaloids in Narcissus tazetta L. subsp. tazetta by HPLC/PDA. J Res Pharm 23:498–505
Kim Y, Hasegawa P, Bressan R (1981) In vitro propagation of hyacinth [Hyacinthus orientalis]. HortScience 16:645–647
Kumar S, Kanwar J, Sharma D (2006) In vitro propagation of Lilium. Adv Hortic Sci 20(2):181–188
Langens-Gerrits M, Alberts M, De Klerk GJ (1998) Hot-water treatment before tissue culture reduces initial contamination in Lilium and Acer. Plant Cell Tiss Org Cult 52:75–77
Langens-Gerrits MM, De Klerk G-JM (1999) Micropropagation of flower bulbs: Lily and Narcissus. Methods Mol Biol 11:141–147
Li SY, Chen C, Zhang HQ, Guo HY, Wang H. Wang L, Zhang X, Hua SN, Yu J, Xiao PG, Li RS, Tan X (2005) Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res 67(1):18–23
Maelicke A (2000) Allosteric modulation of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Dement Geriatr Cogn Disord 11:11–18
Malik S, Cusidó RM, Mirjalili MH, Moyano E, Palazón J, Bonfill M (2011) Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem 46(1):23–34
Malik M, Bach A (2017) High-yielding repetitive somatic embryogenesis in cultures of Narcissus L.‘Carlton’. Acta Sci Pol Hortorum Cultus 16(2):107–112
Muraseva DS, Novikova TI (2018) Efficient protocol for in vitro propagation from bulb scale explants of Fritillaria ruthenica Wikstr. (Liliaceae), a rare ornamental species. Rend Lincei-Sci Fis 29(2):491–497
Nasircilar AG, Mirici S, Karagüzel ÜÖ, Eren Ö, Baktir I (2011) In vitro propagation of endemic and endangered Muscari mirum from different explant types. Turk J Bot 35(1):37–43
Niimi Y, Onozawa T (1979) In vitro bulblet formation from leaf segments of lilies, especially Lilium rubellum Baker. Sci Hortic 11(4):379–389
Ozel CA, Khawar KM, Unal F (2007) In vitro axillary bulblet regeneration of Turkish yellow grape hyacinth (Muscari macrocarpum Sweet) from twin scale explants. Res J Agric Biol Sci 3(6):924–929
Ozel CA, Khawar KM, Unal F (2015) Factors affecting efficient in vitro micropropagation of Muscari muscarimi Medikus using twin bulb scale. Saudi J Biol Sci 22(2):132–138
Pierik R, Steegmans H (1975) Effect of auxins, cytokinins, gibberellins, abscisic acid and ethephon on regeneration and growth of bulblets on excised bulb scale segments of hyacinth. Physiol Plant 34(1):14–17
Podwyszyńska M, Kosson R, Treder J (2015) Polyamines and methyl jasmonate in bulb formation of in vitro propagated tulips. Plant Cell Tiss Org Cult 123(3):591–605
Ptak A, Simlat M, Kwiecień M, Laurain-Mattar D (2013) Leucojum aestivum plants propagated in in vitro bioreactor culture and on solid media containing cytokinins. Eng Life Sci 13(3):261–270
Rahimi Khonakdari M, Mirjalili MH, Gholipour A, Rezadoost H, Moridi Farimani M (2018) Quantification of galantamine in Narcissus tazetta and Galanthus nivalis (Amaryllidaceae) populations growing wild in Iran. Plant Genet Resour 16(2):188–192
Ravishankar G, Ramachandra Rao S (2000) Biotechnological production of phyto-pharmaceuticals. J Biochem Mol Biol Bioph 4:73–102
Rice L, Finnie J, Van Staden J (2011) In vitro bulblet production of Brunsvigia undulata from twin-scales. S Afr J Bot 77(2):305–312
Roy M, Liang L, Xiao X, Feng P, Ye M, Liu J (2018) Lycorine: a prospective natural lead for anticancer drug discovery. Biomed Pharmacother 107:615–624
Sage DO, Lynn J, Hammatt N (2000) Somatic embryogenesis in Narcissus pseudonarcissus cvs. Golden Harvest and St. Keverne. Plant Sci 150(2):209–216
Sairam R, Franklin G, Hassel R, Smith B, Meeker K, Kashikar N, Parani M, Abed DA, Ismail S, Berry K (2003) A study on the effect of genotypes, plant growth regulators and sugars in promoting plant regeneration via organogenesis from soybean cotyledonary nodal callus. Plant Cell Tiss Org Cult 75(1):79–85
Santos J, Santos I, Salema R (1998) In vitro production of bulbs of Narcissus bulbocodium flowering in the first season of growth. Sci Hortic 76(3–4):205–217
Santos I, Salema R (2000) Promotion by jasmonic acid of bulb formation in shoot cultures of Narcissus triandrus L. Plant Growth Reg 30(2):133–138
Sarmadi M, Karimi N, Palazón J, Ghassempour A, Mirjalili MH (2019) Improved effects of polyethylene glycol on the growth, antioxidative enzymes activity and taxanes production in a Taxus baccata L. callus culture. Plant Cell Tiss Org Cult 137(2):319–328
Sellés M, Bergoñón S, Viladomat F, Bastida J, Codina C (1997) Effect of sucrose on growth and galanthamine production in shoot-clump cultures of Narcissus confusus in liquid-shake medium. Plant Cell Tiss Org Cult 49(2):129–136
Slabbert M, De Bruyn M, Ferreira D, Pretorius J (1993) Regeneration of bulblets from twin scales of Crinum macowanii in vitro. Plant Cell Tiss Org Cult 33(2):133–141
Sochacki D (2008) The use of ELISA in the micropropagation of virus-free Narcissus. In: X international symposium on flower bulbs and herbaceous perennials, vol 886, pp 253–258
Staikidou I, Watson S, Harvey BM, Selby C (2005) Narcissus bulblet formation in vitro: effects of carbohydrate type and osmolarity of the culture medium. Plant Cell Tiss Org Cult 80(3):313–320
Stanilova MI, Molle ED, Yanev SG (2010) Galanthamine production by Leucojum aestivum cultures in vitro. In: Cordel GA (ed) The alkaloids: chemistry and biology, 68. Elsevier Inc, Amsterdam, pp 167–270
Sutlana J, Sutlana N, Siddique M, Islam A, Hossain M, Hossain T (2010) In vitro bulb production in Hippeastrum (Hippeastrum hybridum). J Cent Eur Agric 11(4):469–474
Szlávik L, Gyuris Á, Minárovits J, Forgo P, Molnár J, Hohmann J (2004) Alkaloids from Leucojum vernum and antiretroviral activity of Amaryllidaceae alkaloids. Planta Med 70(9):871–873
Taha LS, Sayed SS, Farahat MM, El-Sayed IM (2018) In vitro culture and bulblets induction of Asiatic hybrid lily ‘red alert’. J Biol Sci 18:84–91
Tarakemeh A, Azizi M, Rowshan V, Salehi H, Spina R, Dupire F, Arouie H, Laurain-Mattar D (2019) Screening of Amaryllidaceae alkaloids in bulbs and tissue cultures of Narcissus papyraceus and four varieties of N. tazetta. J Pharm Biomed Anal 172:230–237
Teixeira da Silva JA, Kulus D, Zhang X, Zeng S, Ma G, Piqueras A (2016) Disinfection of explants for saffron (Crocus sativus) tissue culture. Environ Exp Biol 14:183–198
Thomas TD (2007) Pretreatment in thidiazuron improves the in vitro shoot induction from leaves in Curculigo orchioides Gaertn., an endangered medicinal plant. Acta Physiol Plant 29(5):455–461
Torras-Claveria L, Berkov S, Codina C, Viladomat F, Bastida J (2013) Daffodils as potential crops of galanthamine. Assessment of more than 100 ornamental varieties for their alkaloid content and acetylcholinesterase inhibitory activity. Ind Crops Prod 43:237–244
Trujillo-Chacón LM, Pastene‐Navarrete ER, Bustamante L, Baeza M, Alarcón‐Enos JE, Cespedes‐Acuña CL (2020) In vitro micropropagation and alkaloids analysis by GC–MS of Chilean Amaryllidaceae plants: Rhodophiala pratensis. Phytochem Anal 31(1):46–56
Ulrich MR, Davies FT Jr, Koh YC, Duray SA, Egilla JN (1999) Micropropagation of Crinum Ellen Bosanquet’ by tri-scales. Sci Hortic 82:95–102
Wang L, Du Y, Rahman MM, Tang B, Fan L-J, Kilaru A (2018) Establishment of an efficient in vitro propagation system for Iris sanguinea. Sci Rep 8(1):1–10
Woodruff-Pak DS, Lander C, Geerts H (2002) Nicotinic cholinergic modulation: galantamine as a prototype. CNS Drug Rev 8(4):405–426
Yang X, Huang Y, Fan G (2013) Effects of different photoperiods on in vitro plantlet regeneration of Paulownia plants. J Chem Pharm Res 5(12):1446–1450
Yang L, Wen K-S, Ruan X, Zhao Y-X, Wei F, Wang Q (2018) Response of plant secondary metabolites to environmental factors. Molecules 23(4):762
Zayed R, El-Shamy H, Berkov S, Bastida J, Codina C (2011) In vitro micropropagation and alkaloids of Hippeastrum vittatum. In Vitro Cell Dev Biol Plant 47(6):695–701
Zhang X, Gao J, Peng Z (2013) Callus induction, differentiation and histological observation of Narcissus tazetta var. chinensis floral organs. Forest Res 26(3):320–325
Acknowledgements
The authors thank the Shahid Beheshti University Research Council and the Iran National Science Foundation (INSF, Grant No. 94021603) for financial support of this project. We also thank Fatemeh Goudarzi for her kind collaboration in HPLC analysis.
Author information
Authors and Affiliations
Contributions
MRK contributed to the conception of the study, in vitro cultures establishment, data extraction statistical analysis, and writing of the manuscript. HR helped in HPLC-MS analysis. RH contributed in data collection, statistical analysis and revising the manuscript. MHM supervised the whole experiments and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Ali R. Alan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahimi Khonakdari, M., Rezadoost, H., Heydari, R. et al. Effect of photoperiod and plant growth regulators on in vitro mass bulblet proliferation of Narcissus tazzeta L. (Amaryllidaceae), a potential source of galantamine. Plant Cell Tiss Organ Cult 142, 187–199 (2020). https://doi.org/10.1007/s11240-020-01853-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01853-y