INTRODUCTION
Adenostemma plants are members of the Asteraceae family. These plants are annual or perennial herbs that grow wild, commonly known as sticky daisies. They are widely distributed in tropical regions of Asia, Africa, and America (Koyama, 2002). Adenostemma is frequently reported to have 20–24 species, according to King and Robinson (1974), Porteners (1992), and Bremer et al. (1994). However, Koyama (2002) claimed that there might be more than 30 species of Adenostemma J. R. et G. Forst (tribe Eupatorieae) that have been identified and may be found in Asia, Africa, Australia, America, and several oceanic islands. Common names in Southeast Asia were rumput tahi babi, sumbong gajah, rumput pasir (Malay), daun tempel daging boton (Filipino), and tuyenhung (Vietnamese) (Wiart, 2006).
Most Adenostemma species are small, with erect and branched stems, opposite leaves, and terminal inflorescences with many flowered heads. Their leaves and whole plants have been used in folk medicine to remedy diseases such as fever, inflammation, and lung injury. Numerous studies have been conducted on the chemical composition and bioactivity of Asteraceae plants. Nevertheless, plants in the genus Adenostemma are still generally unknown. Since their purported therapeutic properties have been confirmed by scientific studies, interest in these plants has grown recently. However, no comprehensive review has summarized the medicinal applications, phytochemical characteristics, and pharmacological properties of the species in the genus. Therefore, we thoroughly discuss the subjects and evaluate the scientific data supporting the therapeutic use of Adenostemma in this study.
METHODS
We searched the literature through Google Scholar (https://scholar.google.com/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), ScienceDirect (https://www.sciencedirect.com/), and KNApSAcK (http://www.knapsackfamily.com/KNApSAcK/). We used articles published between 1935 and 2022 due to the scarcity of information on Adenostemma. The search terms used to access the database were “Adenostemma,” “Chemical Compound of Adenostemma,” and “Bioactivities of Adenostemma.” We carefully studied all the obtained literature, and 70 publications were chosen for this review.
RESULTS AND DISCUSSION
Species, distributions, and traditional uses
Adenostemma species, their distribution, and their traditional uses are shown in Table 1. In this article, we describe 10 species of Adenostemma found worldwide, with various local names and traditional uses that have been previously reported.
Adenostemma lavenia (L.) O. Kuntze is the most widely reported species in the genus Adenostemma. Adenostemma lavenia has different local names in different world regions. Adenostemma lavenia (L.) O. Kuntze was recognized by Backer and Bakhuizen van den Brink (1965), who successfully identified Adenostemma in Java, Indonesia. They described A. lavenia as having a glandular-hairy or subglabrous stem, ovate, obtuse, acute apex, dentate, or serrate leaves, and achenes at maturity were densely muricate. Individuals in this species thrive in humid environments, areas with some shade, woodlands, brushwood, ditches, and by the side of the road. Additionally, Tjitrosoedirdjo (2002) identified A. lavenia in Sumatera, Indonesia. The morphological traits were comparable to and relatively consistent with those described by Backer and Bakhuizen van den Brink (1965).
A survey of medicinal plants in North Kalimantan, Indonesia, conducted by Arthur in 1952, found that A. lavenia leaf extract (local name: tomali mali or nonokot) was traditionally used by local people to treat fever and healing after childbirth (Arthur, 2011). The root of this plant is chewed with an areca nut, and its ginger is used as a cough remedy. The crushed leaves can be used to soothe sunburned skin, and the leaves withered over a fire can be used to ripen boils. The leaves can also prevent hair loss by washing the hair with them. Leaves mixed with salt can be used for sore throats (Batubara and Prastya, 2020; Kusumawati et al., 2003).
Adenostemma lavenia may also be found in Southeast Asian regions, such as Thailand (Koyama et al., 2016). However, an A. lavenia with eight varieties was recognized in India and South Asia (Panigrahi, 1975). Mathew and Mathew (1983) identified another species besides A. lavenia in south India, i.e., Adenostemma viscosum. The whole plant of A. lavenia is used against digestive disorders in the Wayanad district of Kerala, India (Prasad et al., 2013). Adenostemma lavenia is also widely distributed in tropical regions of East Asia, such as Japan, Korea, the Taiwan region, and mainland China. Adenostemma lavenia has long been used as an herbal medicine for treating inflammation, edema, pneumonia, and lung congestion in the Taiwan region (Cheng et al., 1979). Leaf or whole plant is usually used by the Red-headed Yao people in Jinping from Yunan Province, China, to treat flu, toothache, falls-related injuries, hepatitis, pneumonia, quinsy, enteritis, lymphadenitis, stomach bug, stomach calculus, and vesical calculus (Long and Li, 2004). Meanwhile, A. lavenia has two varieties in Australia: var. lavenia and var. lanceolatum (Miq.) Koster (Orchard, 2011).
Adenostemma madurense DC, a new species from south India, was published by Panigrahi (1975). However, based on the morphological characteristics of the leaf, peduncle, and achene, Panigrahi (1975) regarded this taxon as a variety of A. lavenia. Koyama (2001) highlighted that A. madurense has broadly ovate leaves, an obtuse leaf margin, and slightly glandular muricate in the upper part of the achene, recognizing it as a separate species. Furthermore, Jeong et al. (2017) reported new distribution records of A. madurense on Jeju Island in Korea. They described that A. madurense is distinguished from A. lavenia by its broad ovate or ovate to oblong leaves, about 15–21 cm long by 7–12 cm wide, and smooth achenes with a small muricate on the upper surface, growing on dry mountain slopes in evergreen forests, while A. lavenia is found in wetland areas and on the edge of ponds. Adenostemma. madurense is primarily distributed in Nepal, Thailand, Japan, and the Taiwan region.
Adenostemma platyphyllum Cass has erect, up to 100 cm tall, petioles up to 8 cm, opposite, broadly ovate up to 18 cm long, and up to 13 cm wide, rough-surfaced leaves, white flowers, and somewhat purple leaves (Blair and Madrigal, 2005; Nurlela et al., 2022b). King et al. (1976) also reported that A. platyphyllum had a chromosome number (n) equal to five. In Ecuador (South America), A. platyphyllum was used as an antitussive, analgesic, and remedy for snake bites and scorpion stings. The three Adenostemma plant species described so far, A. lavenia, A. madurense, and A. platyphyllum, are displayed in Figure 1. They were collected from the Biopharmaca Conservation and Cultivation Station, Tropical Biopharmaca Research Center, IPB University, Bogor, and were identified by the curator of herbarium Bandungense (FIPIA) SITH ITB.
Adenostemma macrophyllum is a large herb 0.3–1 m tall, bears large broadly ovate leaves and adult achenes black and smooth. It may be found mainly throughout Java and Sumatera in Indonesia, Sabah in peninsular Malaysia, and eastern Queensland in Australia (Backer and Bakhuizen van den Brink, 1965; Orchard, 2011; Tjitrosoedirdjo, 2002).
Adenostemma parviflorum was recognized as a distinct taxon (Koster, 1935; Koyama, 2002; Panigrahi, 1975) but was identified as A. lavenia var. parviflorum by Backer and Bakhuizen van den Brink (1965) and Koster (1966). Adenostemma parviflorum differs from other species mainly in its smaller florets and heights between 0.35 and 0.7 m. Its habitats are secondary forests, bushes near rivers, grasslands, and swampy areas, in areas with an altitude of 500–2,000 m. Adenostemma parviflorum is distributed in Thailand, China, the Pacific Islands, Hawaii, Malesia, Sumatra, Java, Kalimantan, Sulawesi, Panay, and New Guinea (Tjitrosoedirdjo, 2002).
Adenostemma viscosum is distinct from A. lavenia, being found from Africa to Sri Lanka, India, Indonesia, and the Pacific as far as Hawai’i, while A. lavenia is restricted to its type of site in Sri Lanka (King et al., 1976). Adenostemma viscosum is widespread in East Africa, such as in the Republic of Kenya, the Republic of Uganda, the Republic of Rwanda, the United Republic of Tanzania, the Republic of Malawi, the Republic of Burundi, and the Republic of Zambia (Kokwaro, 2009). In Sabah, Malaysia, the leaf extract of A. viscosum was used to prevent postpartum infections. At the same time, root decoction is used to treat stomachaches in the Malaysian Peninsula. The stems and leaves of A. viscosum may also be used to cure migraines, sinusitis, vertigo, and headaches (Abuga et al., 2022).
 | Table 1. List of Adenostemma species, distributions, and traditional uses. [Click here to view] |
 | Figure 1. (A) Adenostemma lavenia. (B) Adenostemma madurense. (C) Adenostemma platyphyllum. [Click here to view] |
Another Adenostemma species distributed in the south American region—Argentina and Brazil—is Adenostemma brasilianum. This species lives in low-elevation areas, in the understory of forests or along their borders, in damps, and in partially shaded environments (King and Robinson, 1987; Moraes and Monteiro, 2006).
Adenostemma is a medicinal plant used to control ailments afflicting humans for a long time in Africa (Retief, 2002). For example, in the Kabale district of Uganda, in East Africa, the leaves of Adenostemma caffra DC or Adenostemma caffrum DC were used to cure sore throats and skin wounds (Hamill et al., 2000). In other East African regions, such as Ethiopia, the stem and leaf of Adenostemma perrottetii DC. are used to treat dysentery and snakebite via the oral route (Giday et al., 2010). Adenostemma mauritianum was also used to cure coughs, measles, and chicken pox by making powdered leaves and macerating them in water as an infusion in Nigeria (Ajibesin et al., 2008).
Chemical compounds
Arthur (2011) conducted an earlier study of the chemical composition of the genus Adenostemma in Borneo, Indonesia. A predominance of alkaloids and essential oils was revealed in this study on leaves and the whole plant of A. lavenia by qualitative phytochemical assays. Due to their traditional use being widespread in some regions of the world, A. lavenia is the most studied species for its chemical contents and bioactivities. We summarized the chemical components available in Adenostemma in Table 2.
The chemical constituents in Adenostemma tissues and extracts have been identified and precisely quantified using different methods. In our previous study, we used the spectrophotometry method to quantify the total phenolic content (TPC) and total flavonoid content (TFC) of three species of Adenostemma. The water extract of A. lavenia leaves was reported by that study to possess higher TPC (14.40 mg GAE/g DW) and TFC (4.73 mg QE/g DW) than A. madurense and A. platyphyllum (Nurlela et al., 2022b). It was also revealed in the study that the various morphological characteristics of the species significantly influenced its phenolic and flavonoid compositions. Moncayo et al. (2021) also determined the TPC and TFC of ethanol extract of the aerial parts of A. platyphyllum, which contained TPC and TFC of 9.89 ± 0.02 (mg GAE/g dry extract) and 476.02 ± 12.35 (mg QE/g dry extract), respectively. They also conducted phytochemical screening on A. platyphyllum, showing that the ethanol extract of its aerial parts consisted of flavonoids, saponins, terpenoids, steroids, and tannins.
A rapid and reliable method based on high-performance liquid chromatography (HPLC) has been developed to determine the essential oils in A. lavenia. A total of 0.029% of essential oil was identified in the methanol extract of the branches and leaves of A. lavenia (Huyen et al., 2019). Furthermore, these researchers successfully identified flavonoid compounds, such as quercetin, in another Adenostemma species, i.e., A. viscosum. The methanol extract of the branches and leaves of A. viscosum contained 0.067% ± 0.002% quercetin and 1.24% essential oils.
Fauzan et al. (2018) established a simple and sensitive method, a profiling analysis approach using pyrolysis gas chromatography coupled with a mass spectrometer (Py-GCMS) to characterize the chemical composition of A. lavenia and A. platyphyllum. It was shown in the Py-GCMS results that 125 chemical components were present, including terpenoids, phenolics, alkaloids, and fatty acids. Epoxycyclododecane, 4-allyl-2,6-dimethoxyphenol, cis,cis,cis- 8,11,14-eicosatrienoic acid, tetradecahydroanthracene, and levoglucosan were the five most prominent substances overall. N-bearing compounds, alkaloids, aromatic compounds, terpenoids, and steroids predominated in A. lavenia. However, phenolic compounds and originated lipids (fatty acids) were the most prevalent chemicals in A. platyphyllum.
Several compounds have been successfully isolated from Adenostemma species. Bohlmann and Mahanta (1978) isolated germacrene D (Fig. 2A) and three kaurenoic acid derivatives (Fig. 2B–D) from an entire plant of Adenostemma caffrum extracted with Et2O-petrol. These kaurenoic acid derivatives were identical to the previously isolated compound from the Eupatorium album, another genus from Asteraceae (Herz and Sharma, 1976).
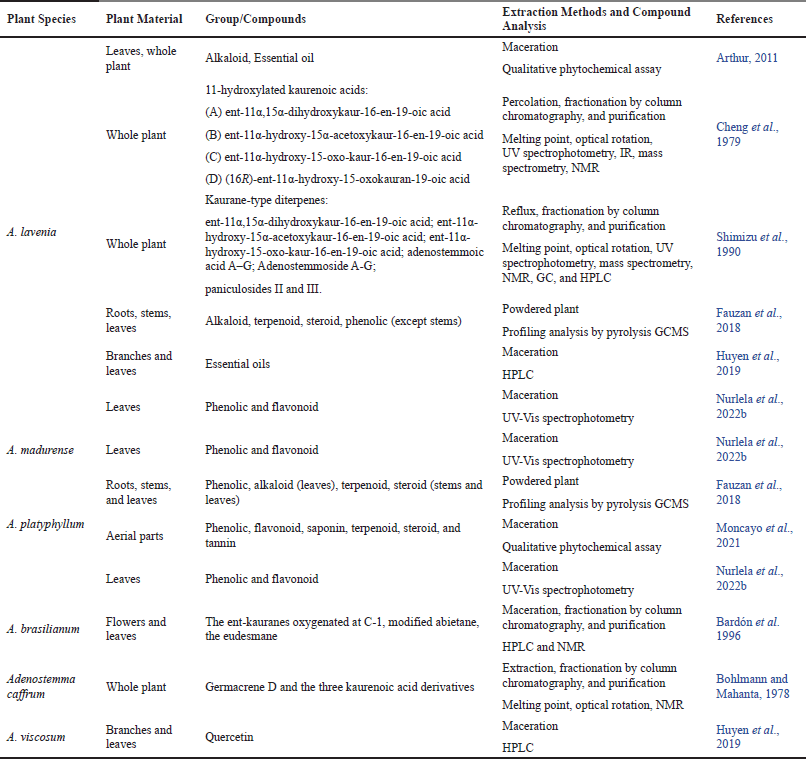 | Table 2. Chemical constituents found in Adenostemma species. [Click here to view] |
Cheng et al. (1979) also isolated four 11-hydroxylated kaurenoic acids from A. lavenia, namely ent-11α-hydroxy-15α-acetoxykaur-16-en-19-oic acid (Fig. 3A), ent-11α,15 α-dihydroxykaur-16-en-19-oic acid (11α,15OH-KA) (Fig. 3B), (16R)-ent-11α-hydroxy-15-oxokauran-19-oic acid (Fig. 3C), and ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic acid (11αOH-KA) (Fig. 3D). The compounds in Figure 2B and C were identical to a previously isolated compound from A. caffrum (Fig. 2B and D). These compounds (Fig. 3A–D) were isolated from a whole plant of A. lavenia extracted with hexane and ethanol.
Furthermore, Shimizu et al. (1990) isolated kaurene-type diterpenes from A. lavenia. Three of the compounds were identical to those that Cheng et al. (1979) had previously reported (Fig. 3A, B, and D). Other 11-oxygenated kauran-19-oic acids were adenostemmoic acids A and B (1, 3) and their glycosides: adenostemmosides A and B (2, 4), paniculosides II and III (5, 6), adenostemmoic acid C-G (7, 9, 11, 13, 15), and their glycosides: adenostemmosides C-G (8, 10, 12, 14, 16), as depicted in Figure 4. These compounds were obtained from the methanolic extract of fresh plants, which were dissolved in water and extracted with ether. Hexane-benzene (1:1) and methanol-water (8:2) were used to partition the ether extract. The isolated compounds were characterized using various instruments, such as a spectrophotometer UV-Visible, spectropolarimeter, mass spectrometer, GC, HPLC, 1H-NMR, and 13C-NMR. In addition to A. lavenia, several ent-kauranes oxygenated at C-1, a modified abietane (Fig. 5A), and eudesmane (Fig. 5B) was also found in the aerial parts of A. brasilianum (Bardón et al., 1996).
 | Figure 2. The compounds isolated from Adenostemma caffrum. [Click here to view] |
 | Figure 3. The structure of 11-hydroxylated kaurenoic acids isolated from A. lavenia. [Click here to view] |
 | Figure 4. The kaurene-type diterpenes isolated from A. lavenia. [Click here to view] |
More recently, Maeda et al. (2022) successfully isolated three kaurenoic acids from another Adenostemma species, i.e., A. madurense, from Japan, and compared them with A. lavenia from Japan and the Taiwan region. They found that Japanese A. lavenia and A. madurense had high amounts of ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic acid (11αOH-KA) and moderate quantities of ent-11α,15 α-dihydroxykaur-16-en-19-oic acid (11α,15OH-KA). In contrast, Taiwanese A. lavenia mostly contained 9,11αOH-KA (adenostemmoic acid B). The bioactivities of the compounds in Adenostemma will be discussed separately in the following section.
 | Figure 5. Abietane and eudesmane isolated from A. brasilianum. [Click here to view] |
Pharmacological investigations
Numerous bioactivity studies of extracts and isolated compounds for their potential antioxidant, anti-inflammatory, and antimelanogenic properties have been motivated by the significant efficacy of Adenostemma species in traditional remedies. Here, we provide an overview of the notable pharmacological investigations of Adenostemma that have been reported in Table 3.
The crude extracts and isolated compounds from leaves, stems, roots, and whole plants of the Adenostemma species demonstrate various biological activities. The leaf has been extensively studied compared to the stem and root. The pharmacological activities discussed in this review include antioxidant, anti-inflammatory, antimelanogenic, antitumor, and antiviral activities.
Antioxidant activity
Antioxidants are substances that can prevent the formation of free radicals through several mechanisms. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are two common forms of radicals. In general, free radicals are produced due to the influence of internal and external factors. External factors include pollution and cigarette smoke, while internal factors include intracellular metabolism (Pizzino et al., 2017; Sharifi-Rad et al., 2020).
ROS and RNS are produced by intracellular metabolism as byproducts of the cellular redox reaction that occurs when adenosine triphosphate is formed to provide energy for the cells by consuming oxygen. When in balance, immunological responses and cellular functions are supported by ROS and RNS. However, unbalanced ROS and RNS concentrations result in oxidative stress, potentially causing chronic and degenerative diseases (Tungmunnithum et al., 2018). Many natural antioxidant compounds have been used as substitutes for synthetic antioxidants in medical and pharmaceutical applications, considering that natural compounds are less toxic (Sharifi-Rad et al., 2020).
Some Adenostemma species have been evaluated for antioxidant activity using several methods. Generally, in vitro assay of antioxidant activity used diphenyl-2-picrylhydrazyl (DPPH) and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) to quantify the radical-scavenging activity or measure ferric reducing antioxidant power and cupric ion reducing antioxidant capacity assay based on redox reaction. The antioxidant activity of the water extract of whole plant A. lavenia was determined using DPPH and ABTS methods, resulting in inhibition percentage, IC50 DPPH, and ABTS of 91.28% ± 1.39%, 121.82 ± 15.84 µg/ml, 3.38 ± 0.17 mg TE/g extract, respectively (Budiarti et al., 2019).
Batubara et al. (2020) reported that the antioxidant activity of A. lavenia leaves reached an IC50 value of 252.02 ± 3.23 µg ml−1, ABTS of 3.63 ± 0.41 mg trolox/g sample (water fraction) and IC50 value of 222.37 ± 1.16 µg ml−1, ABTS of 3.24 ± 0.39 mg trolox/g sample (chloroform fraction). The antioxidant activity in A. platyphyllum has also been investigated, resulting in inhibition percentage and IC50 of 77.50 ± 0.39 and 1.60 mg ml−1, respectively, in the ethanol extract of aerial plant parts (Moncayo et al., 2021).
In addition to radical-scavenging activity, an antioxidant activity assay can be conducted on cellular levels in vitro. In a concentration-dependent way, A. lavenia extracts and ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic acid (11αOH-KA) isolated from A. lavenia CHCl3 leaves extract increased the yeast lifespan and provided Schizosaccharomyces pombe and B16F10 cells with resistance to H2O2. 11αOH-KA stimulated the production of heme oxygenase-1 (HO-1) and the antioxidative transcription factor nuclear factor-E2-related factor 2 (Nrf2), a mammalian homolog of pap1+, in B16F10 cells (Batubara et al., 2020). More recently, Maeda et al. (2022) reported that Nrf2 and HO-1 protein levels were increased by 11αOH-KA, 9,11αOH-KA in a dose-dependent manner but not by 11α,15OH-KA in RAW 264.7 cells. These kaurenoic acids were isolated from the CHCl3 fraction of A. lavenia and A. madurense.
Anti-inflammatory activity
Inflammation is a normal response of the immune system to protect tissues from infection, injury, or disease. Various factors, such as pathogens, noxious substances, damaged cells, and ingested toxins, can trigger such a process (Chen et al., 2018). These factors induce acute or chronic inflammatory reactions in the body that may lead to many illnesses, such as arthritis, heart disease, lung injury, atherosclerosis, metabolic syndrome, allergies, and other autoimmune disorders, and a pathological component of cancer (Chen et al., 2019; Gonçalves and Romano, 2016; Gonçalves and Romano, 2016; Vogl et al., 2013). Inflammation can be activated by various mediators, including cytokines, such as bacterial endotoxins or lipopolysaccharide (LPS), nitric oxide (NO), and prostaglandins, which are produced from the metabolism of arachidonic acid catalyzed by the cyclooxygenase-2 (COX-2) enzyme (Tuwalaid et al., 2022; Wang et al., 2019).
Several investigations have been conducted to assess the anti-inflammatory properties of Adenostemma species. Chen et al. (2019) investigated the anti-in?ammatory effects of ethyl acetate fractions of A. lavenia (EAAL) in vitro and in vivo. These researchers revealed that EAAL decreased proinflammatory cytokine responses, with 4-hydroxycinnamic acid (p-coumaric acid) as its principal constituent. It was also reported in this study that EAAL inhibited COX-2 and protein expression of inducible NO synthase (iNOS), phosphorylation of IκB-α, MAPKs, and AMP-activated protein kinase, activated HO-1, and Nrf2 in LPS-stimulated cells and lung tissues, and antioxidant enzymes (catalase, SOD, and GPx).
 | Table 3. Pharmacological investigation of Adenostemma species. [Click here to view] |
Furthermore, Maeda et al. (2022) demonstrated that isolated kaurenoic acids, 11αOH-KA and 9,11αOH-KA had an anti-inflammatory effect by reducing NO production in LPS-stimulated RAW 264.7 cells, decreasing iNOS protein levels, and suppressing the transcript levels of proinflammatory molecules (IL-6 and TNFα). At the same time, 11α and 15OH-KA did not have any appreciable inhibitory effects.
Recently, Kobayashi et al. (2022) succeeded in separating antimelanogenic, anti-inflammatory, and cytotoxic activities by altering the 19th position of 9,11αOH-KA or adenostemmoic acid B (AB) (carboxy implicated in the avoidance of cytotoxicity). NO synthesis and iNOS expression were suppressed by long-chain alkylation (hydrophobic) without an antimelanogenic effect. At doses greater than 3 μM, AB inhibited NO production in RAW264.7 cells. Surprisingly, NO production was effectively reduced by the hydrophobic derivatives, hexyl and octyl, at lower concentrations of 1 and 0.3 μM, respectively.
Antimelanogenic activity
Melanogenesis, which results in the creation of melanin, the primary pigment responsible for human skin, eye, and hair coloring, is crucial for shielding the skin from UV radiation (D’Mello et al., 2016; Pillaiyar et al., 2018). However, the overproduction of melanin (hyperpigmentation) is linked to various human disorders, such as freckles, melasma, age spots, and pigmented acne scars (Avcil et al., 2021; Kumarasinghe, 2018). Therefore, antimelanogenic compounds are usually used to counter hyperpigmentation. 11OH-KA, which is present in the leaves of Adenostemma and plants from another genus of Asteraceae, such as Pteris and Gochnatia, is a promising candidate for antimelanogenic drugs (Kuroi et al., 2017) excess melanin can be undesirable, particularly on the face where spots or freckles are associated with an appearance of aging. In this study, we found that ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic acid (11α-OH KA).
Melanogenesis is prevented by 11αOH-KA by suppressing tyrosinase gene expression. The conversion of L-tyrosine to L-DOPA (L-3,4-dihydroxyphenylalanine) is catalyzed by tyrosinase, the rate-limiting enzyme in melanin production in melanocytes. L-3,4-dihydroxyphenylalanine is oxidized to DOPA-quinone (Yardman-Frank and Fisher, 2021). Hamamoto et al. (2020) demonstrated the potential of Adenostemma plants for antimelanogenesis in an in vivo study. They discovered that mice treated with an aqueous extract of an A. lavenia leaf containing a high amount of 11αOH-KA had decreased pigmentation in hair. Additionally, 9,11αOH-KA contained in A. lavenia and A. madurense has an antimelanogenic effect by suppressing the expression of the tyrosinase gene (Maeda et al., 2022). Furthermore, the short-chain alkylation of the carboxy group of 9,11-OH-KA increased its antimelanogenic effects. In contrast, NO synthesis and iNOS expression were inhibited by long-chain alkylation, but did not have antimelanogenic effects (Kobayashi et al., 2022).
Other bioactivities
The antiproliferative activities of extracts from A. lavenia and the pharmacological activities mentioned above have been shown in other investigations. Both 11αOH-KA and 9,11-OH-KA were isolated from methanol extracts of entire A. lavenia plants and had antitumor efficacy with low nonspecific cytotoxicity activity against L5178Y leukemia cells. Additionally, the longevity of mice implanted with sarcoma-180 was increased by these substances (Shimizu et al., 1990).
11αOH-KA and 9,11-OH-KA are some diterpenoids found not only in Adenostemma species, but also in other plants, such as Plectranthus asirensis and Pteris semipinnata L. These substances have antioxidant, anti-inflammatory, anticancer, and antitumor activities (Lu et al., 2013; Saeed et al., 2020). Other diterpenoids, such as brianthein V, are isolated from Briareum asbestinum and have cytotoxic and antiviral activities (Coval et al., 1988). It was revealed in a recent study that the expression of the antioxidant protein heme oxygenase (HO-1) could be promoted by 11αOH-KA isolated from A. lavenia through the transcription of factor Nrf2 in mouse melanoma cells (Batubara et al., 2020). The activation mechanism of the HO-1-Nrf2 antioxidant gene is currently suggested as a cellular target for curing COVID-19 sufferers (McCord et al., 2020). The kaurene diterpene glycoside ent-6-11-dihydroxy-15-oxo-16-kauren-19-oic acid β-D-glucopyranosyl ester and adenostemmosides B were exhibited in our recent study about the in silico of some kaurene diterpenoids. Plants in the Adenostemma genus were also found to have inhibitory activities on the nonstructural and structural proteins of SARS-CoV-2 virus (Nurlela et al., 2022a). It was also shown by the investigation that the ligands were firmly bounded to the binding sites of the proteins, achieved stability, were well absorbed by the human intestine and noncarcinogenic substances, and did not lead to DNA changes. We expect that these substances might have promise as preventative and therapeutic agents in the battle against COVID-19 disease.
CONCLUSION
The traditional uses, chemical compounds, and pharmacological studies covered in this review demonstrated that the accumulation of bioactive compounds in various Adenostemma species widely utilized in traditional medicine might account for the observed health-promoting effects. Adenostemma lavenia, A. madurense, and A. platyphyllum were the most frequently reported species in the genus Adenostemma. In recent years, there has been a significant advancement in scientific knowledge of plant chemical compounds and biological activities. However, numerous species in the genus have yet to be thoroughly characterized, suggesting that further investigation is needed. Although several bioactivities of Adenostemma have been investigated, the chemical compounds in Adenostemma may have other bioactivities that still need to be discovered and evaluated. According to the explanation in the current review article, crude extracts or isolated compounds of Adenostemma have various pharmacological properties that have been demonstrated by in vitro, in vivo, and in silico test results, making Adenostemma one of the plants with the potential to be developed in discovering new drugs.
ACKNOWLEDGMENTS
This research was funded by the Directorate General of Higher Education, Research, and Technology, Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia, for Penelitian Disertasi Doktor (PDD) no. 3794/IT3.L1/PT.01.03/P/B/2022. Additionally, this research was supported by the bilateral exchange program directorate general of higher education/Japan society for promoting the science joint research project 2022, ongoing no. 023.17.1. 690439/2022.
AUTHORS CONTRIBUTIONS
All of the authors have made a significant contribution to this manuscript. Concept and design were done by IB and HT. Data acquisition and analysis/interpretation were done by NN and B. Drafting manuscript is done by NN. Critical manuscript revision is carried out by IB, WN, AI, and NN. Supervision and final approval is done by IB. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abuga I, Sulaiman SF, Abdul Wahab R, Ooi KL, Abdull Rasad MSB. Phytochemical constituents and antibacterial activities of 45 Malay traditional medicinal plants. J Herb Med, 2022; 32:100496. CrossRef
Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA. Ethnobotanical survey of Akwa Ibom State of Nigeria. J Ethnopharmacol, 2008; 115(3):387–408. CrossRef
Arthur HR. A phytochemical survey of some plants of North Borneo. J Pharm Pharmacol, 2011; 6(1):66–72. CrossRef
Avcil M, Akman G, Klokkers J, Jeong D, Çelik A. Clinical efficacy of dissolvable microneedles armed with anti-melanogenic compounds to counter hyperpigmentation. J Cosmet Dermatol, 2021; 20(2):605–14. CrossRef
Bardón A, Montanaro S, Catalán CAN, Díaz JG, Herz W. Kauranes and related diterpenes from Adenostemma brasilianum. Phytochemistry, 1996; 42(2):479–84. CrossRef
Backer CA, Bakhuizen van den Brink Jr RC. Flora of Java, II. N.V.P. Nordhoff, Groningen, Netherland, 1965.
Batubara I, Astuti RI, Prastya ME, Ilmiawati A, Maeda M, Suzuki M, Hamamoto A, Takemori H. The antiaging effect of active fractions and Ent-11α-Hydroxy-15-Oxo-Kaur-16-En-19-Oic acid isolated from Adenostemma lavenia (L.) O. Kuntze at the cellular level. Antioxidants, 2020; 9(8):719. CrossRef
Batubara I, Prastya, ME. Potential use of Indonesian medicinal plants for cosmetic and oral health: a review. J Kimia Valensi, 2020; 6(1):118–32.
Blair S, Madrigal B. Plantas antimaláricas de Tumaco: Costa Pacífica Colombiana. Univ. de Antioquia, Medellín, Colombia, 2005.
Bohlmann F, Mahanta PK. Kaurenic acid derivatives from Adenostemma caffrum. Phytochemistry, 1978; 17:814–5. CrossRef
Bremer K, Anderberg AA, Karis PO, Lundberg J. Asteraceae: cladistics & classification. Timber Press, Portland, OR, 1994.
Budiarti E, Batubara I, Ilmiawati A. Potensi beberapa ekstrak tumbuhan asteraceae sebagai antioksidan dan antiglikasi. J Jamu Indo, 2019; 4(3):103–11. CrossRef
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 2018; 9(6):7204–18. CrossRef
Chen JJ, Deng JS, Huang CC, Li PY, Liang YC, Chou CY, Huang GJ. p -Coumaric-acid-containing Adenostemma lavenia ameliorates acute lung injury by activating AMPK/Nrf2/HO-1 signaling and improving the antioxidant response. Am J Chin Med, 2019; 47(07):1483–506. CrossRef
Cheng PC, Hufford CD, Doorenbos NJ. Isolation of 11-Hydroxyated kauranic acids from Adenostemma lavenia. J Nat Prod, 1979; 42(2):183–6. CrossRef
Coval SJ, Cross S, Bernardinelli G, Jefford CW. Brianthein V, a new cytotoxic and antiviral diterpene isolated from Briareum asbestinum. J Nat Prod, 1988; 51(5):981–4. CrossRef
D’Mello SAN, Finlay GJ, Baguley BC, Askarian-Amiri ME. Signaling pathways in melanogenesis. Int J Mol Sci, 2016; 17(7):1144. CrossRef
Fauzan A, Praseptiangga D, Hartanto R, Pujiasmanto B. Characterization of the chemical composition of Adenostemma lavenia (L.) Kuntze and Adenostemma platyphyllum Cass. IOP Conf Ser Earth Environ Sci, 2018; 102:012029. CrossRef
Giday M, Asfaw Z, Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J Ethnopharmacol, 2010; 132(1):75–85. CrossRef
Girardi C, Butaud JF, Ollier C, Ingert N, Weniger B, Raharivelomanana P, Moretti C. Herbal medicine in the Marquesas Islands. J Ethnopharmacol, 2015; 161:200–13. CrossRef
Gonçalves S, Romano A. The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind Crops Prod, 2016; 83:213–26. CrossRef
Gumisiriza H, Sesaazi CD, Olet EA, Kembabazi O, Birungi G. Medicinal plants used to treat “African” diseases by the local communities of Bwambara sub-county in Rukungiri District, Western Uganda. J Ethnopharmacol, 2021; 268:113578. CrossRef
Hamamoto A, Isogai R, Maeda M, Hayazaki M, Horiyama E, Takashima S, Koketsu M, Takemori H. The high content of Ent-11α-hydroxy-15-oxo-kaur- 16-en-19-oic acid in Adenostemma lavenia (L.) O. Kuntze leaf extract: with preliminary in vivo assays. Foods, 2020; 9(1):73. CrossRef
Hamill FA, Apio S, Mubiru NK, Mosango M, Bukenya-Ziraba R, Maganyi OW, Soejarto DD. Traditional herbal drugs of southern Uganda, I. J Ethnopharmacol, 2000; 70(3):281–300. CrossRef
Herz W, Sharma RP. New hydroxylated ent-kauranoic acids from Eupatorium album. J Org Chem, 1976; 41(6):1021–6. CrossRef
Huyen DN, Tuyen PT, Loan VT, Dac LX. The contents of 20-hydroxyecdysone (20e), quercetin and essential oils in Asteraceae species growing in Tam Dao district, Vinh Phuc province, Vietnam. J Forest Sci Technol, 2019; 7:72–81.
Jeong KS, Heo TI, Lee KH, Choi K, Kim HJ. A new record of Adenostemma madurense DC. (Asteraceae) in Korea. Korean J Plant Res, 2017; 30(3):331–4.
Kalema J, Ssegawa P. The flora of highly degraded and vulnerable wetland ecosystems of Nyamuriro and Doho, Uganda. African J Ecol, 2007; 45(s1):28–33. CrossRef
King RM, Kyhos DW, Powell AM, Raven PH, Robinson H. Chromosome numbers in Compositae. XIII. Eupatorieae. Ann Mo Bot Gard, 1976; 63(4):862. CrossRef
King RM, Robinson H. Studies in the Eupatorieae (Asteraceae). CXXVII. Additions to the American and Pacific Adenostemmatineae. Adenostemma, Gymnocoronis and Sciadocephala. Phytologia, 1974; 29:1–20. CrossRef
King RM, Robinson H. The genera of the Eupatorieae (Asteraceae). Monographs in Systematic botany from the Missouri Botanical Garden 22. Missouri Botanic Garden, St Louis, MO, 1987.
Kobayashi T, Tanaka N, Suzuki M, Maeda M, Batubara I, Iswantini D, Koketsu M, Hamamoto A, Takemori H. Adenostemmoic acid B suppresses NO production by downregulating the expression and inhibiting the enzymatic activity of iNOS. Phytochem Lett, 2022; 49:131–7. CrossRef
Kokwaro JO. Medicinal plants of East Africa. III. University of Nairobi Press, Nairobi, Kenya, 2009
Koster JT. The Compositae of the Malay archipelago. Blumea, 1935; 1:351–536.
Koster JT. The compositae of New Guinea. 1. Nova Guinea Bot, 1966; 24:497–614.
Koyama H. Adenostemma lavenia and its allies (Compositae) in Japan, China and Thailand. Mem Nat Sci Mus Tokyo, 2001; 37:159–68.
Koyama H. Taxonomic studies in the compositae of Thailand 14. tribe eupatorieae. Bull Nat Sci, 2002; 28(2):49–60.
Koyama H, Sukhonthip B, Pimwadee P, DJ Nicholas H. Compositae, Asteraceae. In: Thawatchai S, Balslev H (Eds.). Flora of Thailand. Forest Herbarium, Royal Forest Department, Bangkok, Thailand.
Kumarasinghe P. Pigmentary skin disorders. Springer International Publishing, Cham, Switzerland, 2018. CrossRef
Kuroi A, Sugimura K, Kumagai A, Kohara A, Nagaoka Y, Kawahara H, Yamahara M, Kawahara N, Takemori H, Fuchino H. The importance of 11α-OH, 15-oxo, and 16-en moieties of 11α-Hydroxy-15-oxo-kaur-16-en-19-oic acid in its inhibitory activity on melanogenesis. Skin Pharmacol Physiol, 2017; 30(4):205–15. CrossRef
Kusumawati I, Djatmiko W, Rahman A, Studiawan H, Ekasari W. Eksplorasi keanekaragaman dan kandungan kimia tumbuhan obat di hutan tropis gunung Arjuno. J Bahan Alam Indonesia, 2003; 2(3):100–4.
Larsen K. Spices. In: de Guzman CC, Siemonsma JS (Eds.). PROSEA. Plant resources of Southeast Asia 13. Backhuis Publishers, Leiden, Netherland, 1999.
Long C, Li R. Ethnobotanical studies on medicinal plants used by the Red-headed Yao People in Jinping, Yunnan Province, China. J Ethnopharmacol, 2004; 90(2–3):389–95. CrossRef
Lu Y, Wu K, Li L, He Y, Cui L, Liang N, Mu B. Characterization and evaluation of an oral microemulsion containing the antitumor diterpenoid compound ent-11alpha-hydroxy-15-oxo-kaur-16-en-19-oic-acid. Int J Nanomedicine, 2013; 1879–86. CrossRef
Maeda M, Suzuki M, Fuchino H, Tanaka N, Kobayashi T, Isogai R, Batubara I, Iswantini D, Matsuno M, Kawahara N, Koketsu M. Diversity of Adenostemma lavenia, multi-potential herbs, and its kaurenoic acid composition between Japan and Taiwan. J Nat Med, 2022; 76(1):132–43. CrossRef
Mathew PM, Mathew A. Studies on the South Indian Compositae. V. Cytotaxonomic consideration of the tribes Vernonieae and Eupatorieae. Cytologia, 1983; 48(3):679–90. CrossRef
McCord JM, Hybertson BM, Cota-Gomez A, Geraci KP, Gao B. Nrf2 activator PB125® as a potential therapeutic agent against COVID-19. Antioxidants, 2020; 9(6):518. CrossRef
Moncayo S, Cornejo X, Castillo J, Valdez V. Preliminary phytochemical screening for antioxidant activity and content of phenols and flavonoids of 18 species of plants native to western Ecuador. Trends Phytochem Res, 2021; 5(2):92–104.
Moraes MD, Monteiro R. A familia Asteraceae na planície litorânea de Picinguaba, Ubatuba, São Paulo. Hoehnea, 2006; 33:41–78.
Nurlela N, Awaluddin F, Batubara I, Wahyudi ST. Computational study of kaurene diterpenoids for antivirals against SARS-CoV-2. J App Pharm Sci, 2022a; 12(8):112–29. CrossRef
Nurlela N, Nurfalah R, Ananda F, Ridwan T, Ilmiawati A, Nurcholis W, Takemori H, Batubara I. Variation of morphological characteristics, total phenolic, and total flavonoid in Adenostemma lavenia, A. madurense, and A. platyphyllum. Biodiversitas, 2022b; 23(8) :3999–4005. CrossRef
Omoigui ID. Cytomorphological studies of some Nigerian Asteraceae (Compositae)—tribe Eupatorieae. Niger J Life Sci, 2015; 5(1):160–7. CrossRef
Orchard A. A review of Australian Adenostemma J. R. Forst. & G. Forst. (Asteraceae: Eupatorieae). Telopea, 2011; 13(1–2):341–8. CrossRef
Panigrahi G. The genus Adenostemma (Compositae) in the Indian region. Kew Bull, 1975; 30(4):647. CrossRef
Pillaiyar T, Namasivayam V, Manickam M, Jung S. Inhibitors of melanogenesis: an updated review. J Med Chem. 2018; 61(17):7395–418. CrossRef
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev, 2017; 8416763:1–13. CrossRef
Porteners MF. Adenostemma. In: Harden GJ (Ed.). Flora of New South Wales, III. Kensington. University of NSW Press, New South Wales, Australia, pp 148–149, 1992.
Prasad AGD, Shyma TB, Raghavendra MP. Plants used by the tribes for the treatment of digestive system disorders in Wayanad district, Kerala. J Appl Pharm Sci, 2013; 3(08):171–5.
Rahminiwati M, Iswantini D, Takemori H, Koketsu M, Sianipar R, Novalia R, Achmadi SS, Sjahriza A, Soebrata BM, Wulanawati A. Indonesian medicinal plants with anti-inflammatory properties and potency as Chronic Obstructive Pulmonary Disease (COPD) Herbal Medicine. Pharmacogn J, 2022; 14(4):432–44. CrossRef
Retief E. The tribe Eupatorieae (Asteraceae) in Southern Africa. In: Zachariades C, Muniappan R, Strathie LW. In Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata, Durban, South Africa, 23–25 October 2000. ARC-PPRI, Pretoria, South Africa, 2002.
Saeed AAM, Ali AM, Fdhel TA. HPLC-ESI-MS analysis of some bioactive substances in two yemeni medicinal plants. Electron J Univ Aden Basic Appl Sci, 2020; 1(4) :225–35. CrossRef
Sharifi-rad M, Kumar NVA, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Fokou PVT, Azzini E, Peluso I, Mishra AP, Nigam M, El Rayess Y, El Beyrouthy M, Polito L, Iriti M, Martins N, Martorell M, Docea AO, Setzer WN, Calina D, Cho WC, Sharifi-rad J. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol, 2020; 11:1–21. CrossRef
Shimizu S, Miyase T, Umehara K, Ueno A. Kaurane-type diterpenes from Adenostemma lavenia O. Kuntze. Chem Pharm Bull, 1990; 38(5):1308–12. CrossRef
Tjitrosoedirdjo SS. Notes on The Asteraceae of Sumatera. Biotropia, 2002; 19 :65–84.
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel), 2018; 5(3):93. CrossRef
Tuwalaid B, Iswantini D, Tri Wahyudi S. Potential Adenostemma lavenia and Muntingia calabura extracts to inhibit cyclooxygenase-2 activity as a therapeutic strategy for anti-inflammation: experimental and theoretical studies. Indones J Chem, 2022; 22(1):754. CrossRef
Vogl S, Picker P, Mihaly-Bison J, Fakhrudin N, Atanasov AG, Heiss EH, Wawrosch C, Reznicek G, Dirsch VM, Saukel J, Kopp B. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J Ethnopharmacol, 2013; 149(3):750–71. CrossRef
Wang T, Fu X, Chen Q, Patra JK, Wang D, Wang Z, Gai Z. Arachidonic acid metabolism and kidney inflammation. Int J Mol Sci, 2019; 20(15):3683. CrossRef
Wiart C. Medicinal plants of the Asia-Pacific: drugs for the future? World Scientific Publishing, Toh Tuck Link, Singapore, 2006 CrossRef
Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: from ultraviolet radiation to stem cells. Exp Dermatol, 2021; 30(4):560–71. CrossRef