
January 2023 152
NEWS BSBI
BSBI ADMINISTRATION
President MICHELINE SHEEHY SKEFFINGTON ‘Seagal’, Ballynacourty, Clarinbridge, Co. Galway, Ireland
Hon. General Secretary and BSBI Company Secretary
Chair of the Trustees
Membership Secretary (Payment of subscriptions and changes of address) and BSBI
News distribution
Hon. Field Meetings Secretary (including enquiries about field meetings)
Panel of Referees & Specialists (comments and/or changes of address)
BSBI News – Editor
British & Irish Botany – Editorin-Chief
BSBI Chief Executive
BSBI Finance Manager (all financial matters except Membership)
BSBI Communications Officer (including publicity, outreach and website; British & Irish Botany)
BSBI Fundraising Manager (including donations, legacies, grants and organisational support)
BSBI Head of Science
BSBI Scientific and England Officer (& V.c. Recorders – comments and/ or changes of address)
BSBI Scotland Officer
STEVE GATER
28 Chipchase Grove, Durham DH1 3FA
CHRIS MILES
Braeside, Boreland, Lockerbie DG11 2LL
GWYNN ELLIS
41 Marlborough Road, Roath, Cardiff, CF23 5BU Please quote membership number on all correspondence; see address label on post.
JONATHAN SHANKLIN
11 City Road, Cambridge CB1 1DP
MARTIN RAND (with assistance from Jo Parmenter)
3 Kings Close, Chandler’s Ford, Eastleigh SO53 2FF
JOHN NORTON
215 Forton Road, Gosport PO12 3HB
IAN DENHOLM
3 Osier Close, Melton, Woodbridge IP12 1SH
JULIA HANMER
65 Sotheby Road, London N5 2UP
JULIE ETHERINGTON
Church Folde, 2 New Street, Mawdesley, Ormskirk L40 2QP
LOUISE MARSH
SARAH WOODS
23 Bank Parade, Otley LS21 3DY
KEVIN WALKER
Suite 14, Bridge House, 1–2 Station Bridge, Harrogate HG1 1SS
PETE STROH
c/o Cambridge University Botanic Garden, 1 Brookside, Cambridge CB2 1JE
MATT HARDING
c/o Royal Botanic Garden, Inverleith Row, Edinburgh EH3 5LR
BSBI Ireland Officer PAUL GREEN
Yoletown, Ballycullane, New Ross, Co. Wexford, Y34 XW62, Ireland
BSBI Countries Support Manager JAMES HARDING-MORRIS
BSBI Training Coordinator (FISC and Identiplant)
20 Manchester Square, New Holland, Barrowupon-Humber DN19 7RQ
CHANTAL HELM
BSBI Database Officer TOM HUMPHREY
BSBI Book Sales Agent
PAUL O’HARA
Summerfield Books, The Old Coach House, Skirsgill Business Park, Penrith, CA11 0DJ
BSBI website: bsbi.org BSBI News: bsbi.org/bsbi-news
micheline.sheehy@nuigalway.ie
Tel. 00 353 91 790311
steve.gater@bsbi.org
Tel. 07823 384083
chris.miles01@btinternet.com
Tel. 01576 610303
gwynn.ellis@bsbi.org
Tel. 02920 332338
fieldmeetings@bsbi.org
Tel. 01223 571250
VC11recorder@hantsplants.net
Tel. 07531 461442
john.norton@bsbi.org
Tel. 02392 520828
bib@bsbi.org
Tel. 07725 862957
julia.hanmer@bsbi.org
Tel. 07757 244651
julie.etherington@bsbi.org
Tel. 07944 990399
louise.marsh@bsbi.org
Tel. 07725 862957
sarah.woods@bsbi.org
Tel. 07570 254619
kevin.walker@bsbi.org
Tel. 01423 858327 or 07807 526856
peter.stroh@bsbi.org
Tel. 01832 720327 or 01223 762054
matt.harding@bsbi.org
Tel. 07814 727231
paul.green@bsbi.org
Tel. 00 353 87 7782496
james.harding-morris@bsbi.org
Tel. 07526 624228
chantal.helm@bsbi.org
Tel. 07896 310075
tom.humphrey@bsbi.org
info@summerfieldbooks.com
Tel. 01768 210793
CONTENTS January 2023 No. 152
Including welcome to new staff, reports from the 2022 AGM and BSBI conference, meetings update,
Cover photo: Toothwort (Lathraea squamaria), Sheepleas, Surrey, March 2022. Chris Heath. One of the images submitted in the Spring category for the 2022 BSBI Photographic Competition. See pp. 60 & 80

Contributions for future issues should be sent to the Editor, John Norton (john.norton@bsbi.org)
The Botanical Society of Britain and Ireland (BSBI) is the leading charity promoting the enjoyment, study and conservation of wild plants in Britain and Ireland. BSBI is a company limited by guarantee registered in England and Wales (8553976) and a charity registered in England and Wales (1152954) and in Scotland (SC038675). Registered office: 28 Chipchase Grove, Durham, DH1 3FA. All text and illustrations in BSBI News are copyright and no reproduction in any form may be made without written permission from the Editor. The views expressed by contributors to BSBI News are not necessarily those of the Editor, the Trustees of the BSBI or its committees. BSBI ©2022 ISSN 0309-930X

FROM THE PRESIDENT / EDITORIAL 1 ARTICLES Changes to the heath vegetation of the Wirral Peninsula Eric Greenwood 3 The flora of a suburban garden and change over time Tony F. Marshall 11 Callitriche palustris (Narrow-fruited Waterstarwort): a Cumbrian annus mirabilis Jeremy Roberts 18 Two early records of the rare Rumex rupestris (Shore Dock) David Pearman 23 Modelling the history of Pyrola minor (Common Wintergreen) over 200 years in Berwickshire (v.c. 81) Michael Braithwaite 25 Ivy confusions? Alison Rutherford 28 Caltha palustris var. radicans: overlooked and under-recorded? Richard Lansdown & Tim Pankhurst 31 Take the risk – do a FISC! Sarah Whild & Sue Dancey 34 BEGINNER’S CORNER Making sense of mouse-ears Mike Crewe 37 ADVENTIVES & ALIENS Adventives & Aliens News 28 Compiled by Matthew Berry 41 Does Doronicum × longeflorens occur in Britain and Ireland? Erik Christensen 49 Artemisia austroyunnanensis Y. Ling & Y.R. Ling (‘Giant Mugwort’) in v.c.16 (West Kent) Rodney Burton 51 Printed in the UK by Henry Ling Ltd, Dorchester on FSC™ certified paper using ink created
renewable materials.
with
Cicerbita macrophylla subsp. macrophylla (Blue Sow-thistle) in v.c. 64 (M.W. Yorkshire) Howard Beck 53 Dispersal of Oxalis corniculata (Procumbent Yellow-sorrel) Rodney Burton 54 NOTICES
Panel of VCRs, Plant Atlas launch schedule, BSBI Photographic Competition and contents of British & Irish Botany 4:3 56 COUNTRY ROUNDUPS Botanical news from around England, Wales, Scotland and Ireland Compiled by Pete Stroh 62 OBITUARIES Compiled by Chris Preston 74 REVIEWS Compiled by Clive Stace 76 Importing PDF into Indesign Select “Show Import Options” when importing and choose “Crop to: Trim” and tick box for “Transparent Background”. This just includes the white, no arrows etc. The white box denotes the clear space the logo must have around it. Be careful when placing the logo that the white box doesn’t obscure anything. The logo below is at the minimum size allowed so cannot be reduced further but could be enlarged if appropriate, but the white background must be enlarged by the same amount too.
FROM THE PRESIDENT
Well,as I said at the Annual Conference in November, it’s like buses. We wait for decades for a female president (Mary Briggs, the first in 1998) – and then two come along at once! I hope to learn from them both in my presidency. But, even more incredible, I am only the second president from the Republic of Ireland … after D. A. Webb, who is also a hard act to follow. One thing I aim to do from the start is to put Ireland more on the map and in May 2023, we will host the Annual Summer Meeting in Killarney. We have a vibrant young group involved and it’s shaping up well with Atlantic oak woodlands, bogs, lakes, possibly islands, to explore – and maybe mountain exploring for the more intrepid.
One thing Ian Denholm was already keen to do as president, is to bring together different societies together, preferably in the field. We both hit on collaborating with the Royal Entomological Society to organise a one-day outing to Daneway Banks Reserve where the Large Blue butterfly resides. It’s now happening in June. I am keen to emphasise management of sites for conservation, but often, management for plants can be detrimental to invertebrates; the structure of a sward is at least as important. And of course, butterflies, like many invertebrates, feed on different plants in their larval stage. Daneway Banks is small and because we will be in the Large Blue season, it is limited to 30 people –sadly that means just 15 per society. However, I
EDITORIAL
AsI write this on New Year’s Eve it’s pouring with rain for the second day running, but I’m still looking forward to getting out over the next couple of days to take part in the BSBI’s New Year Plant Hunt. As usual I will concentrate on the built up habitats (walls, pavements, kerbs and urban grass verges), which usually prove to be the most fruitful places for finding plants in flower at this time of year – and hopefully it should be relatively dry underfoot. I hope the awful winter weather will soon abate so that we can all look forward to seeing the winter annuals
have a plan to initiate a similar event in Ireland with our National Parks & Wildlife Service that might be a two-day event in 2024. So watch this space!
We were all delighted to meet up at last at the Natural History Museum in November and 2023 promises well with lots of field excursions and events. My aim is to attend as much as I can. But I have resolved not to fly where at all possible. So I am very grateful for the offer to keep me on zoom for now for the meetings. That frees me up to visit each country for at least one field meeting over the next two years –including the Channel Islands and hopefully a return visit to the Isle of Man.
Of course March 2023 is filled with the Atlas launch. There has been huge build-up to this truly magnum opus. It has much more information this time, including analysis of trends which should inform conservation practitioners. For what are we doing all this recording for, if not to be of benefit to the conservation of our flora and habitats? This should become central to all our activities in future years. But it’s essential still to keep monitoring in the field and contribute locally where threats are perceived. In the meantime, here’s to a great launch and fabulous read for many years to come!
Micheline Sheehy Skeffington michelinesheehy@gmail.com
(which more typically flower during February and March in mild winters) as well as the early spring flowers which help brighten up botanising in the early part of the year. In this regard Mike Crewe’s Beginners Corner article on Mouse-ears (Cerastium spp.) (p. 37 ), might come in useful. I hope you enjoy this and the other articles in this issue and I wish members happy plant-hunting in 2023.
John Norton john.norton@bsbi.org
2 BSBI NEWS 152 | January 2023 FROM THE PRESIDENT / EDITORIAL
Changes to the heath vegetation of the Wirral Peninsula
 ERIC GREENWOOD
ERIC GREENWOOD
The Wirral peninsula (v.c. 58, Cheshire) extends for 27 km (17 miles) north-west of Chester. It is 8–13 km (5–8 miles) wide and is bounded on the north-east side by the Mersey estuary, on the south-west side by the Dee estuary and on the northwest side by the Irish Sea. Running parallel to the Mersey and Dee estuaries are two low sandstone ridges. Nearest to the Mersey are Bidston, Oxton and Storeton heaths, whilst on the Dee side there are Grange Hill (West Kirby), Caldy, Irby and Thurstaston Commons and Heswall Common. Large portions of these heaths were extant in 2022 but Heswall is fragmented into the Beacons, the Dales and Cleaver Heath with smaller fragments at Poll Hill, Whitfield Common and elsewhere. As their names suggest heaths refer to flat land on acid soils supporting ericaceous dwarf shrubs such as Calluna vulgaris (Heather). Within this dominant vegetation there may be variation with wetter and drier parts and even localised mire and flushed areas. In areas with deeper soils farms were established within the heath, e.g. Benty Farm at Thurstaston or Dale Farm
Above: Bidston Heath in 2022 from near where Ellis took his photo (see p. 5). The trees have completely hidden the mill. Barbara Greenwood
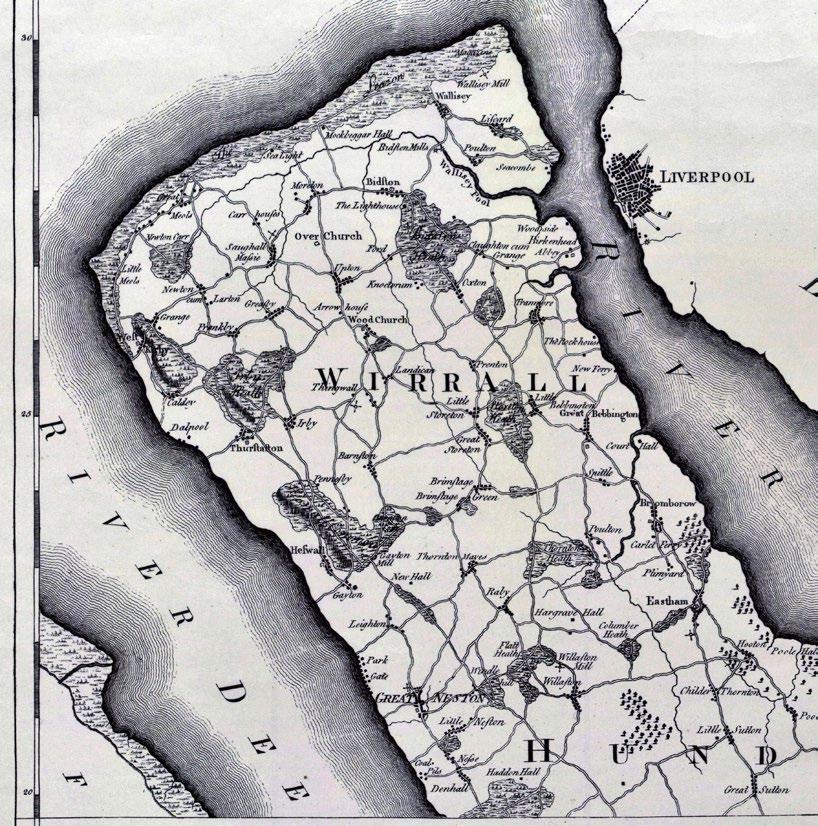
at Heswall. Burdett’s map of Wirral (1777, Figure 1) shows the heaths criss-crossed by tracks.
In addition to those on the sandstone outcrops other heaths in the south-east of the peninsula include Thornton Common and Flatt Heath, Willaston, but Burdett’s map indicates further fragments around Brimstage, Neston and Eastham. These heaths were situated on boulder clay overlying sandstone but so far as is known there were no rock exposures. Leaching of the basic clay must have occurred over many years to give rise to heaths, or alternatively the heaths developed on less calcareous glacial sands and gravels. Anderson (2021) described a similar situation with the development of heaths on the limestone plateau of the Peak District.
Whatever the substrate, heaths have an anthropogenic origin as grazed common lands. Without grazing they would be deciduous woodland
BSBI NEWS 152 | January 2023 3
Changes to the heath vegetation of the Wirral Peninsula
(see below). I am unaware of any detailed historical surveys of Wirral heaths but it is likely that the sequence of events follows a similar pattern to those in southern England. Following the retreat of the ice after the last ice age, woodland covered much of lowland England. From about 5000 BP Neolithic farmers started to make clearings in the forest and as time passed these clearances became larger. The thin acid soils on the sandstone of Wirral probably supported a fairly open birch-oak woodland possibly
with some pine. The land would have been grazed from earliest times with the woodland changing to heath. Continued grazing maintained the heath vegetation (Webb, 1986). After most woodland had been reclaimed for farms, by Anglo-Saxon times the acid heaths remained as open common land. The local population had rights to the common, most importantly pasturage, the rights to graze domestic animals and possibly estover, the right to gather wood or herbage (bracken and gorse).
4 BSBI NEWS 152 | January 2023
Figure 1. Burdett’s map of Wirral, part of his map of Cheshire 1777. Reproduced courtesy of Cheshire History Association.
During the 18th century there was considerable pressure to enclose the commons for various purposes, ranging from improving the lands for farming or even for building towns and villages. Nearby Liverpool may have developed in this way (Hosking & Stamp, 1963). Details of the enclosure of the Wirral heaths is not certain but there are enclosure awards for Thornton (1815), Heswall (1859) and Thurstaston (1883) at the Chester Record Office. According to Hosking & Stamp (1963) rights of grazing and pasture still existed for part of Thurstaston Common in the 1960s.

At some stage grazing ceased on all the Wirral heaths and commons but there is no record of when this happened. In recent years limited conservation grazing has been introduced to parts of Thurstaston Common. On other areas, e.g. Oxton Common, which is now a golf course, some cutting of the heath (in the roughs) is practised. Heswall Dales and Cleaver Heath are managed as nature reserves with management limited to preventing scrub encroachment.
The vegetation
Early Liverpool floras (Hall, 1839; Dickinson, 1851) list the heath species found from the early 19th century but gave no indication of the vegetation types present. Green (1933) and Newton (1971) provide lists of some of the characteristic or notable species. Green divides his lists into dry and wet heaths and into Bidston/Oxton and Heswall/ Thurstaston groups. By then many changes had already taken place so that they were unable to describe the flora and vegetation that the earliest botanists had encountered (Hall, 1839; Dickinson, 1851). However, it is possible to define four plant communities that were still present in the 1970s and 80s when Rodwell (1991a, b) and his collaborators did their field work. These communities were H9
Calluna vulgaris-Deschampsia flexuosa heath; H8 Calluna vulgaris-Ulex gallii heath; M16 Erica tetralix-Sphagnum compactum wet heath and W16 Quercus spp.-Betula spp.-Deschampsia flexuosa woodland.
H9 and H8 are typical dry heaths whilst M16 is usually wet throughout the year. It is likely these
and variations of these, existed in the early 19th century along with flushed communities, which have now disappeared. Over the years floristic diversity has decreased with the loss of rarer and perhaps characteristic species. In particular, the species known to have occurred on or near the heaths must have existed in flush communities with some base enrichment. What was known as the ‘Pinguicula field’ at the Raby Mere end of Thornton Common, well-known to local botanists (Green, 1902), was probably a stream-side flush. Pinguicula vulgaris and Carex pulicaris were typical of flushed communities and to a lesser extent so was Carex echinata
In addition to the open heath communities, woodlands also covered part of the sandstone ridges. These are now more extensive than in the 19th century. Rodwell (1991a) identified these as W16 Quercus spp.-Betula spp.-Deschampsia flexuosa woodlands. They are characteristic of the warm and dry south-eastern lowland zone of British vegetation. On Wirral, birch species are commoner than oak but identifying the species is more difficult. Both Betula pendula (Silver Birch) and B. pubescens (Downy Birch) are perhaps equally frequent, with numerous intermediates or hybrids. Both Quercus robur (Pedunculate Oak) and Q. petraea (Sessile Oak) are present but most oaks are the hybrid Q. × rosacea Rodwell (1991a) recognises two sub-communities: Quercus robur (W16a) and Vaccinium myrtillus-Dryopteris dilatata (W16b). He recorded the presence of the Quercus robur sub-community on Wirral but on some
BSBI NEWS 152 | January 2023 5
Bidston Mill and heath c. 1900. Photo taken from Green (1933), which was first published in his Flora of 1902. The photograph was taken by J.W. Ellis, probably in the 1890s.
Changes to the heath vegetation of the Wirral Peninsula
of the Wirral heaths large parts of the ground flora are dominated by Dryopteris dilatata (Broad Buckler-fern) suggesting that the Vaccinium myrtillusDryopteris dilatata sub-community characteristic of more oceanic conditions is also present. The range of vegetation types found on Wirral heaths are at the north-western edge of their range in Britain (Rodwell, 1991a, b) and at the northern edge of the lowland zone as defined by Rackham (2003).
Floristic changes
Without accurate data it is difficult to measure change. Nevertheless, the 19th century Liverpool floras recorded the observations of local botanists. Much of their work was brought together by De Tabley with his own observations up to about 1875. His Flora of Cheshire was updated by his son and published in 1899. In 1902 Green published a Flora of the Liverpool District based on the observations of the Liverpool Naturalists’ Field Club during the previous eight or nine years. This gave detailed localities and the names of recorders. In 1933 he published a new edition but this gave much less precision. New records were incorporated into this edition but it is not clear to what extent old records were verified as still being present.
In 1971 Newton published his Flora of Cheshire based on his own records and those of his collaborators. A supplement was published in 1990. De Tabley, Green and Newton all devoted paragraphs describing the Wirral heath flora. Since 1990 records were published online in the BSBI database.
Given these limitations, tables have been prepared showing the known losses up to 1933 (Table 1) and since 1933 (Table 2.) Table 3 lists heath species that are thought to still occur on the Wirral heaths, with a summary of all three tables presented in Table 4.For each species, selected attributes (Hill et al., 2004) are given together with their IUCN threat status in England (Stroh, et al., 2014). Overall, the heath flora is characteristic of nutrient poor soils and climatically is typical of northern temperate areas of Europe that do not extend eastwards far into Asia. The heaths comprise both wet and dry areas.
Comparison with recent studies
Comparing the attributes of the lost species with those currently found on the heaths, the overall characteristics seem little different. The geographical affinities are very similar. If climatic change was taking affect it might be in the area of oceanicity, but the flora does not appear to be getting any less or more oceanic. However, it does appear that species requiring a wetter substrate are being lost and remaining species need less wet conditions. This was one of the conclusions reached by Ash et al. (2021) in their 39-year study of fixed quadrats on Thurstaston Common. They also suggested that there was some evidence for nutrient enrichment, because species currently have a preference for more fertile conditions. This could be from atmospheric deposition but also through changes induced by invading birch (Ashburner & McAllister, 2013; Mitchell et al., 1997, 2007, 2018).
Perhaps the most surprising observation in this study is that conditions appeared to have got more acidic in the first half of the 20th century with the loss of species requiring some base enrichment but with no increase in acidity after the 1930s. Sandstone soils are acid lacking bases but it is possible that springs arose where boulder clay was in juxtaposition with the acid sandstone. Similar conditions must have been present on the eastern side of Thurstaston Common where similar species were recorded. Until recently, flushed areas were present on the Dales (part of what was once Heswall Common) but none now survive.
Anderson (2021) in her study of upland grasslands in the Peak District also observed an increasing tendency to acidification and suggested that this might be due to acid rain caused by atmospheric pollution with sulphur and nitrogen oxides from nearby industrial areas. However, recent evidence suggests that the acid rain peak has passed and there is evidence of soils becoming less acidic since the late 1990s (Mitchell et al., 2018). The evidence from the Wirral heaths appears to confirm this trend.
The tables also show the current threat level (based on recent population status) in England (Stroh et al., 2014). This shows that the most threatened
6 BSBI NEWS 152 | January 2023
Changes
to the heath vegetation of the Wirral Peninsula
BSBI NEWS 152 | January 2023 7
Species Threat level F R N E1 E2 Carex echinata (Star Sedge) NT 8 3 2 5 3 Carex pulicaris (Flea Sedge) NT 7 5 2 7 2 Eleocharis acicularis (Needle Spike-rush) NT 10 7 5 5 6 Eleogiton fluitans (Floating Club-rush) LC 11 4 2 8 1 Eriophorum vaginatum (Hare’s-tail Cottongrass) LC 8 2 1 2 6 Filago germanica (Common Cudweed) NT 4 6 4 8 3 Genista anglica (Petty Whin) VU 5 3 2 7 1 Hypericum elodes (Marsh St John’s-wort) NT 10 3 2 7 1 Linum radiola (Allseed) VU 7 4 2 7 3 Littorella uniflora (Shoreweed) LC 10 5 3 7 2 Lycopodiella inundata (Marsh Clubmoss) EN 9 2 1 5 3 Lysimachia minima (Chaffweed) EN 7 5 3 7 3 Omalotheca sylvatica (Heath Cudweed) EN 6 4 3 5 4 Oreopteris limbosperma (Lemon-scented Fern) LC 6 4 3 7 3 Persicaria minor (Small Water-pepper) LC 8 5 8 7 5 Pilularia globulifera (Pillwort) VU 10 4 2 7 2 Pinguicula vulgaris (Common Butterwort) VU 8 6 2 4 6 Potamogeton polygonifolius (Bog Pondweed) LC 10 4 2 7 2 Sagina subulata (Heath Pearlwort) NT 6 6 4 7 3 Sedum anglicum (English Stonecrop) LC 3 4 2 7 1 Selaginella selaginoides (Lesser Clubmoss) LC 7 6 2 4 6 Average 11.7 4.4 2.7 5.9 3.1 No. threatened = 13 (62%)
Table 1. Extinct species 1933 or before. Key to columns is shown after Table 4.
Species Threat level F R N E1 E2 Carex arenaria (Sand Sedge) LC 3 5 2 7 3 Carex demissa (Common Yellow-sedge) LC 8 4 2 5 6 Carex hostiana (Tawny Sedge) LC 9 6 2 7 3 Carex leporina (Oval Sedge) LC 7 5 4 5 4 Coeloglossum viride (Frog Orchid) VU 4 6 2 4 6 Drosera intermedia (Oblong-leaved Sundew) VU 9 2 1 7 2 Drosera rotundifolia (Round-leaved Sundew) NT 9 2 1 5 6 Eleocharis multicaulis (Many-stalked Spikerush) LC 9 4 1 7 2 Gentiana pneumonanthe (Marsh Gentian) NT 7 4 1 7 4 Huperzia selago (Fir Clubmoss) LC 6 2 2 2 6 Pedicularis sylvatica (Lousewort) VU 8 3 2 7 3 Polygala serpyllifolia (Heath Milkwort) NT 7 2 2 7 2 Scutellaria minor (Lesser Skullcap) LC 9 4 2 8 2 Trifolium ornithopodioides (Bird’s-foot Clover) LC 6 5 3 8 2 Average 7.2 3.8 1.9 6.1 3.6 No. threatened: 5 (45%)
Table 2. Extinct species post 1933.
Changes to the heath vegetation of the Wirral Peninsula
Changes to the heath vegetation of the Wirral Peninsula
8 BSBI NEWS 152 | January 2023
Species Threat level F R N E1 E2 Agrostis capillaris (Common Bent)* LC 5 4 4 5 4 Anthoxanthum odoratum (Sweet Vernalgrass) LC 6 4 3 6 4 Avenella flexuosa (Wavy Hair-grass)* LC 5 2 3 5 3 Betula pubescens (Downy Birch) LC 7 4 4 5 4 Betula pendula (Silver Birch) LC 5 4 4 5 4 Betula × aurata (Hybrid Birch) – – – – – –Blechnum spicant (Hard Fern) LC 6 3 3 7 3 Calluna vulgaris (Heather)* NT 6 2 2 5 3 Campanula rotundifolia (Harebell) NT 4 5 2 5 6 Carex binervis (Green-ribbed Sedge) LC 6 3 2 7 1 Carex nigra (Common Sedge)* LC 8 4 2 5 4 Carex pilulifera (Pill Sedge)* LC 5 3 2 7 3 Ceratocapnos claviculata (Climbing Corydalis) LC 5 4 5 7 1 Cytisus scoparius (Broom)* LC 5 4 4 7 3 Danthonia decumbens (Heath Grass) LC 6 4 2 7 3 Digitalis purpurea (Foxglove) LC 6 4 5 8 2 Dryopteris affinis subsp. affinis (Golden-scaled Male-fern) LC 6 5 5 7 3 Dryopteris dilatata (Broad Buckler-fern)* LC 6 4 5 7 3 Dryopteris filix-mas (Male-fern) LC 6 5 5 7 6 Epilobium montanum (Broad-leaved Willowherb) LC 6 6 6 7 3 Erica cinerea (Bell Heather)* NT 5 2 2 7 1 Erica tetralix (Cross-leaved Heath)* NT 8 2 1 7 2 Eriophorum angustifolium (Common Cottongrass)* VU 9 4 1 3 6 Festuca ovina (Sheep’s-fescue)* LC 5 4 2 5 5 Galium saxatile (Heath Bedstraw)* LC 6 3 3 7 2 Hieracium vagum (a hawkweed) LC – – – – –Holcus mollis (Creeping Soft-grass)* LC 6 3 3 7 3 Hypericum pulchrum (Slender St John’s-wort) LC 5 4 3 7 2 Hypochaeris radicata (Cat’s-ear)* LC 4 5 3 8 3 Juncus bulbosus (Bulbous Rush)* LC 10 4 2 5 3 Juncus effusus (Soft-rush)* LC 7 4 4 8 3 Juncus squarrosus (Heath Rush)* LC 7 2 2 7 2 Lonicera periclymenum (Honeysuckle)* LC 6 5 5 8 2 Lotus corniculatus (Common Bird’s-foottrefoil) LC 4 6 2 8 5 Luzula campestris (Field Wood-rush) LC 4 5 2 7 3 Luzula multiflorum subsp. congesta (Heath Wood-rush)* LC 6 3 3 3 6 Lythrum portula (Water-purslane) LC 9 5 3 7 3 Molinia caerulea (Purple Moor-grass)* LC 8 3 2 5 4 Nardus stricta (Mat-grass)* NT 7 3 2 5 3 Narthecium ossifragum (Bog Asphodel)* LC 9 2 1 5 1 Pinus sylvestris (Scots Pine)* – 6 2 2 4 5 Populus tremula (Aspen) LC 5 5 6 5 5
Table 3. Extant species 2021. Key to columns is shown after Table 4.
* Thurstaston Common species listed by Newton (1971).
Table 4. Average values for PLANTATT attributes taken from tables 1–3.
Key to tables
England Red List threat levels: VU = Vulnerable; EN = Endangered; NT = Near threatened; LC = Least concern.
Attribute (Hill et al., 2004):
F = Moisture: 1 = extreme dryness, 7 = constantly wet, 12 = submerged.
R = Reaction: 1 = extremely acid, 9 = calcareous, high pH soils.
N = Fertility: 1 = extremely infertile, 9 = extremely rich or near polluted.
Geographical affinities: E1 = Major biogeographic element, major biome. 1 = arctic-montane, 9 = Mediterraneanatlantic; E2 = Biogeographic, eastern limit. 1 = oceanic, 5 = Eurasian, 6 = circumpolar.
species were already lost by 1933, whilst today very few threatened species remain on Wirral heaths. This suggests that the heaths have lost the majority of the most notable species that made them particularly significant. If, as seems likely, the main reason for the losses is the gradually increasing dryness of the heaths and the absence of grazing with the accompanying invasion by birch scrub, then longterm reasons need to be sought for this change, which seems to be continuing. At present there is no satisfactory explanation.
Discussion
This study suggests that most of the rarer species characteristic of Wirral heaths were lost before 1900 or not long afterwards. The primary cause of this loss appears to be the long-term drying out of the heaths together with management changes. At present it is not clear what is causing the drying out; possibly complex factors involving ground-water hydrology. The heaths were common land used for grazing animals and for the gathering of herbage. These management changes enabled the spread of oak-
BSBI NEWS 152 | January 2023 9 Potentilla erecta (Tormentil)* NT 7 3 2 5 4 Pteridium aquilinum (Bracken)* LC 5 3 3 7 6 Quercus petraea (Sessile Oak)* LC 6 3 4 7 3 Quercus robur (Pedunculate Oak) LC 5 5 4 7 3 Quercus × rosacea (Hybrid Oak) – – – – – –Salix caprea (Goat Willow) LC 7 7 7 5 5 Salix cinerea subsp. oleifolia (Grey Willow) LC 8 6 5 5 4 Salix repens var. repens (Creeping Willow) NT 7 6 3 5 4 Senecio sylvaticus (Heath Groundsel) LC 5 5 6 7 3 Solidago virgaurea (Goldenrod) NT 5 4 3 5 5 Sorbus aucuparia (Rowan)* LC 6 3 4 4 1 Trichophorum germanicum (Common Deergrass)* LC 8 2 1 4 6 Ulex europaeus (Gorse) LC 5 5 3 7 1 Ulex gallii (Western Gorse)* LC 6 3 2 7 1 Vaccinium myrtillus (Bilberry) LC 6 2 2 4 4 Average 6.2 3.8 3.2 6.0 3.4
(18%)
No. threatened = 9
F R N E1 E2 % threatened Extinct before 1933 (21 spp.) 11.7 4.4 2.7 5.9 3.1 62 Extinct post 1933 (13 spp.) 7.2 3.8 1.9 6.1 3.6 36 Extant 2021 (53 spp.) 6.2 3.8 3.2 6.0 3.4 18
Table 3 (cont.)
Changes to the heath vegetation of the Wirral Peninsula
Changes to the heath vegetation of the Wirral Peninsula

birch woodland but primarily the increase in birch species. Photographic and anecdotal evidence suggests that the development of birch woodland is accelerating, invading not only the dry heath but also the remaining fragments of wet heath (see photographs). This change is probably too far advanced for any conservation measures to reverse the process (Mitchell et al., 1997, 2007). The resultant woodland may, in time, not be too dissimilar to the oak-birch woodland from which the heaths were originally derived, where patches of more open habitats were probably maintained by large herbivores and early anthropogenic clearances (Vera, 2000), which would have enabled species of more open heath habitats to survive or flourish.
Acknowledgements
Thanks are due to Barbara Greenwood and Professor Rob Marrs for comments and checking the script and to Dr Hilary Ash and members of Wirral Wildlife for botanical data.
References
Anderson, P. 2021. Peak District. The New Naturalist Library. HarperCollins, London.
Ash, H.J., Brockbank, A., Greenwood, B., Sixsmith, M., Baker-Schomer, M. & Marrs, R.H. 2021. Long-term monitoring of a heathland conservation project: a tale of twelve quadrats. Field Studies Journal 1: 1–23. fsj.fieldstudies-council.org (accessed 11/8/2022).
Ashburner, K. & McAllister, H.A. 2013. The genus Betula. Kew Publishing, Kew.
De Tabley, W. 1899. The Flora of Cheshire. Longmans, Green and Co. London.
Dickinson, J. 1851. The Flora of Liverpool. John van Voorst, Deighton and Laughton, Liverpool.
Green, C.T. 1902. The Flora of Liverpool District. D. Marples & Co., Liverpool.
Green, C.T. 1933. The Flora of Liverpool District. T. Buncle & Co., Arbroath.
Hall, T.B. [1839]. A Flora of Liverpool. Whitaker & Co., London.
Hill, M.O., Preston, C.D. & Roy, D.B. 2004. PLANTATT. Centre for Ecology & Hydrology, Abbots Ripton.
Mitchell, R.J., Marrs, R.H., LeDuc, M.G. & Auld, M.H.D. 1997. A study of succession on lowland heaths in Dorset, southern England: changes in vegetation and soil chemical properties. Journal of Applied Ecology 34: 1426–1444.
Mitchell, R.J., Campbell, C.D., Chapman, S.J., Osler, G.H.R., Vanbergen, A.J., Ross, L.C., Cameron, C.M. & Cole, L. 2007. The cascading effects of birch on heather moorland: a test for the top – down control of an ecosystem engineer. Journal of Ecology 95: 540–550.
Mitchell, R.J., Hewison, R.L., Fielding, D.A., Fisher, J.M., Gilbert, D.J., Hurskainen, S., Pakemann, R.J., Potts., J.M. & Riach, D. 2018. Decline in atmospheric sulphur deposition and changes in climate are the major drivers on long-term change in grassland plant communities in Scotland. Environmental Pollution 235: 956–964.
Newton, A. 1971. Flora of Cheshire. Cheshire Community Council Publications Trust Limited, Chester.
Rackham, O. 2003. Ancient Woodland. Castlepoint Press, Dalbeattie.
Rodwell, J.S. (ed) 1991a. British Plant Communities, vol. 1 Woodlands. Cambridge University Press, Cambridge.
Rodwell, J.S. (ed) 1991b. British Plant Communities, vol. 2 Mires and Heaths. Cambridge University Press, Cambridge.
Stroh, P.A., Leach, S.J., August, T.A., Walker, K.J., Pearman, D.A., Rumsey, F.J., Harrower, C.A., Fay, M.F., Martin, J.P., Pankhurst, T., Preston, C.D. & Taylor, I. 2014. A Vascular Plant Red List for England. Botanical Society of Britain and Ireland, Bristol.
Vera, F.W.M. 2000. Grazing Ecology and Forest History. CABI Publishing, Wallingford.
Webb, N. 1986. Heathlands. The New Naturalist. Collins. London.
Eric F. Greenwood
We are sad to report that Eric Greenwood died in October 2022. He was one of the longest serving members of the Society and an Honorary Member since 1976. His obituary will appear in a future issue.
John Norton, Editor
10 BSBI NEWS 152 | January 2023
Wet heath invaded by birch at Thurstaston, 2022. Eric Greenwood
The flora of a suburban garden and change over time
TONY F. MARSHALL
The garden and its history Gardens can be very biodiverse. Over time I have recorded nearly 1,300 species other than plants in my garden, some of them uncommon in the surrounding area – fungi, invertebrates and a few vertebrates. However, gardens differ from other ‘natural’ habitats in several ways. They are generally more sheltered with a slightly warmer microclimate, they tend to be small (but this is countered by the fact that separate gardens are often clustered together in close contiguity), and they are idiosyncratically managed according to the whim and aims of the gardener, typically involving the introduction of alien species and garden cultivars. Each is usually composed of several microhabitats, often each one small in extent, which may prevent (along with the owner’s selective management) the effective establishment of sustainable ecosystems, but can certainly create high biodiversity per unit area. Most microhabitats can support substantial wildlife, with the exception of extensive paving or oft-mowed lawns (although these can support different plants and creatures in their own right). Lawns, if not assailed by chemical moss-killers or fertilisers, can be excellent for fungi, although this is only realised if mowing is abandoned over autumn.
In the case of my garden, in the Chilterns near Great Missenden (v.c. 24), it is typically sheltered, but the largest part is on the shady colder north side of the house, and I have not noticed that any plants survive there that would not do so in the wild. It is certainly small (300 sq. m. in total), which does limit its capacity for the diversity and extent of microhabitats. Much of it is shaded by a tall hedge and acts essentially as a woodland floor, maintaining a number of true woodland species, but its size means that it cannot possibly rival the diversity or ecology of true woodland. In contact with similarly managed gardens its influence and wildlife value
might be much wider, but unfortunately none of the contiguous gardens are at all favourable to wildlife. The current habitats are three meadow areas (each left uncut until after flowers have seeded, but each with its own character), a hawthorn hedge with much holly and ivy (uncut and higher than neighbouring hedges; it is much used by birds), two mature trees – a Betula pendula (Silver Birch) which was a naturally occurring first-year seedling in 1983 and an older Salix caprea (Goat Willow), scrub, paving (which provides interstitial niches for certain plants), rockery and ‘scree’, and an area occasionally dug over (not often enough!), and side-passages that are shadier and moister than other areas, one supporting a series of ferns. There are also small wood piles and a marshy hollow that was once a pond, created in 1983, until the plastic liner perished, although it is still ‘watered’ by a hose extending from a waterbutt. Surrounding fences make access difficult for larger mammals, but we have had Foxes, Hedgehogs, Squirrels and Muntjac (not to mention Brown Rats) on occasion.
The soil is thin humus over slightly acidic sandy clay with flint pebbles, far from ideal for ‘proper’ gardening. Vegetable crops grow very poorly.
It is not particularly managed for aesthetic purposes, although the largest meadow can be attractive in early spring to early summer with a succession of flowers from bulb plants through Primula veris (Cowslip), P. vulgaris (Primrose) and Hyacinthoides non-scripta (Bluebell) until Silene dioica (Red Campion) and Allium triquetrum (Threecornered Garlic) take over, soon after which it is mown. Another meadow is best in early summer with Hypochaeris radicata (Cat’s-ear), Crepis vesicaria (Beaked Hawk’s-beard), Leucanthemum vulgare (Oxeye Daisy), Rumex acetosa (Common Sorrel) and Rhinanthus minor (Yellow-rattle), plus 19 species of Poaceae, but I have waited in vain so far for a more diverse flora to arrive
BSBI NEWS 152 | January 2023 11 The
flora of a suburban garden and change over time
The flora of a suburban garden and change over time
(Fritillary), Myosotis sylvatica (Wood Forget-menot) and Caltha palustris (Marsh-marigold).

naturally: a meadow requires much patience and a long life! (While seeding may artificially increase diversity in the short term I find that the variety soon dwindles and, in a small area, soon becomes dominated by a few species best suited to the soil and climate.) This second meadow is mossy and contains four waxcap fungi, but they have yet to expand their population to put on a really great
show. A waxcap grassland takes as long to develop as a flower meadow, nature moving on a slower course than our human lives.
My primary aim is to provide a rich wildlife habitat with, as far as possible, a wide variety of plants. I prefer to leave the garden to its own devices (both from design and lack of time, or call it laziness), as it is interesting to observe the succession. Some

12 BSBI NEWS 152 | January 2023
Plate 1. Corner of one meadow beside scrub, April, with Primula veris (Cowslip) (including red variety), Cardamine pratensis (Cuckooflower), Hyacinthoides non-scripta (Bluebell) and Taraxacum (Dandelion). Photographs by the author.
Plate 2. Another meadow in April, by former pond (top right) with native Narcissus pseudonarcissus (Wild Daffodil) going over, Cowslip emerging, Fritillaria meleagris
Plate 3. Same meadow as Plate 2 and wood edge, in June just before cutting, including Dryopteris filismas (Male-fern), Corylus avellana (Hazel), Euphorbia amygdaloides Wood Spurge, Betula pendula (Silver Birch), Silene dioica (Red Campion), Leucanthemum vulgare (Oxeye Daisy), Ranunculus repens (Meadow Buttercup), Hypericum androsaemum (Tutsan), Hesperis matronalis (Dame’s-violet), Allium roseum (Rosy Garlic) and A. triquetrum (Three-cornered Garlic).

Plate 4. Same meadow as shown in Plate 1, June, with Oxeye Daisy, Rumex acetosa (Common Sorrel), Crepis vesicaria (Beaked Hawk’s-beard) and a multitude of grass species.
action, however, is necessary if it is not to become a uniform thicket of scrub and tall grass. I have never used artificial chemicals or fertilisers. Each meadow is mown once a year, the cuttings taken off. Other than that the main pursuit is cutting back over-exuberant shrubs and other plants, of which we seem to have a lot, filling a green bin once a fortnight. (This is a good time to look out for galls,
leaf-mines and micro-fungi, incidentally.) The willow is pollarded every three or four years – I leave it in between because twice it has been visited by egglaying Purple Emperors. Each winter I intend to dig over the ‘cultivated’ area, but recent drought and the hard stony clay have made that very difficult. Occasional seeding or planting is undertaken when opportunity provides, but usually of native plants or

BSBI NEWS 152 | January 2023 13
The flora of a suburban garden and change over time
The flora of a suburban garden and change over time
aliens known to become naturalised, except for some exotic bulbs to provide early spring interest before our natives appear. I would like to renew the pond, but my age prevents such an undertaking. Spilled bird-seed is an occasional source of alien plants.
I took over the garden in 1982, when it was only one year old. Two years later I carried out the first botanical survey, which has been supplemented by casual observations and occasional fuller surveys until the latest complete survey in 2022. Altogether 316 different plant taxa have been recorded, with 198 (63%) currently present.
The flora
(1)Naturally occurring natives
These make up 43% of species currently in the garden. Of the 158 species ever recorded, just over half still occur (85). There have been two major causes of losses in the garden. One is the demise of our pond, with the loss of five plants such as Callitriche (Water-starwort) and Lemna species (duckweeds). The other is the spread of shrubby plants and the loss of open regularly-forked soil, reducing the habitat for arable annuals, of which 21 species have been lost, like Lysimachia arvensis (Scarlet Pimpernel), Papaver rhoeas (Common Poppy) and, most notably, Linaria repens and Chaenorhinum minus (Pale and Small Toadflax). Two species appear to have been lost by converting short grass areas to wildflower meadows: Medicago lupulina (Black Medick) and Galium saxatile (Heath Bedstraw); and two more to the general drying out of the environment due to gradual climate change: Gnaphalium uliginosum (Marsh Cudweed), lost after 2008, and Solanum dulcamara (Bittersweet), lost after 2018. The majority of losses (43), however, remain unexplained, including all three Sonchus spp. (Sow-thistles), Trifolium pratense (Red Clover), Stellaria graminea (Lesser Stitchwort) and Plantago major (Greater Plantain), some of which still grow in the road verge immediately outside my garden, although one might speculate that some losses, such as lesser stitchwort, are due to soil nutrification by air-borne pollution which has reduced numbers of acidophilic plants generally in the region over the same period.
Most of the naturally occurring natives, as might be expected, spread naturally within the garden. Just seven remain present without showing signs of expanding their range. Two of these are recent arrivals, Iris foetidissima (Stinking Iris) and a Taxus baccata (Yew) seedling. One has only limited habitat availability, Typha latifolia (Bulrush). Polygonum aviculare (Knotgrass), Lonicera periclymenum (Honeysuckle) and Ribes uva-crispa (Gooseberry), not planted but naturally occurring, have been stable over a long period. The remaining species is the unexplained arrival of Des Etang’s St John’s-wort Hypericum × desetangsii in 2016, which has remained in situ now for six years, despite the garden never having hosted any other non-shrubby Hypericum
The most persistent spreaders in this group, which require regular removal are Geum urbanum (Wood Avens), Rubus spp. (Bramble) (mostly Rubus vestitus), Urtica dioica (Common Nettle), Hedera helix subsp. helix (Ivy), Carex sylvatica (Wood Sedge) and Geranium robertianum (Herb-Robert). It seems likely that all these are responding to the general nutrification of the local environment from air pollution. Perhaps the most surprising is Wood Sedge, which is happy, like the Wood Avens, to move from its natural shaded environment to just about anywhere in the garden.
More welcome natives, which spread more moderately, are Cardamine pratense (Cuckooflower), Fumaria officinalis (Common Fumitory), Leucanthemum vulgare (Oxeye Daisy), Hyacinthoides non-scripta (Bluebell), Lotus corniculatus (Common Bird’s-foottrefoil) (the native variety, not the more vigorous agricultural import), Primula veris (Cowslip), P. vulgaris (Primrose), and their naturally arising hybrid Primula × polyantha (often more vigorous than its parents and long-lasting). All these occur commonly in the surrounding local area. Of special interest is Geum × intermedium, a hybrid that arose in situ between Wood Avens and Geum rivale (Water Avens) that I planted beside the pond and is not native to the local area. The latter gradually disappeared, apparently hybridised out, while the hybrid flourishes alongside its other parent. The most recent additions to the naturally occurring native list are three plants that only arrived in 2022: Bromopsis ramosa (Hairy Brome),
14 BSBI NEWS 152 | January 2023
a seedling Fagus sylvatica (Beech) and Torilis japonica (Upright Hedge-parsley). Why they took so long, I do not know.
(2)Planted natives
These are native species that I have deliberately introduced at one time or another. They only constitute 14% of the total species present. Once introduced, their survival rate is quite high, twothirds still being present (68%). Of the 13 losses, seven are accounted for by the loss of the pond and therefore the disappearance of suitable habitat. Two of the others show a local preference for more calcareous soil and may have found our clay less to their liking – Briza media (Quaking-grass) and Silene vulgaris (Bladder Campion), neither are at all common in the immediate vicinity either. Another species was affected by its situation becoming overshaded and general drying out – Asplenium trichomanes (Maidenhair Spleenwort). It has similarly been lost from many of its native habitats locally. It is less clear why the three remaining planted natives failed to survive – Ajuga reptans (Bugle), Myrrhis odorata (Sweet Cicely) and Anemone nemorosa (Wood Anemone). All three survived several years and then suddenly failed to re-appear. Lack of moisture might be the reason for Bugle and Wood Anemone, while Sweet Cicely is one of those plants that just come and go anyway (it has disappeared from its only natural site locally, from which I took the seed).
On the other hand, quite a lot of the planted natives (64%) not only survived but are inclined to spread and may require control. The most vigorous seem to be Filipendula ulmaria (Meadowsweet) (which only grows locally by two other ponds where it was also introduced), Helleborus foetidus (Stinking Hellebore), Origanum vulgare (Wild Marjoram), Papaver cambricum (Welsh Poppy), Rhinanthus minor (Yellow-rattle) (but only within one meadow area), Silene dioica (Red Campion) (locally uncommon in natural habitats but can become a nuisance where introduced!), Galium verum (Lady’s Bedstraw), Carex otrubae (False Fox-sedge), Hypericum androsaemum (Tutsan) and Carex pendula (Pendulous Sedge) (a real thug, impossible to remove, a species that is
also increasing in local woodlands to the detriment of other flora and my biggest regret in causing its introduction to my garden). Apart from Carex pendula, none of these plants inclined to take over the garden are very common in more natural habitats. Two ‘native’ bulb plants also spread, but more gradually, Fritillaria meleagris (Fritillary) and Narcissus pseudonarcissus (Wild Daffodil). The original five fritillaries are now 12 flowering plants in 2022 (after 36 years!), plus about the same number that did not flower that year, while the 10 daffodils are now nearly 150 in 20 years. This contrasts with non-native daffodils that show no ability to spread at all, including Narcissus obvallaris (Tenby Daffodil) that remains established only in its original numbers.
(3)Naturally occurring aliens
Many species that invite themselves into our gardens, whether we want them or not, are aliens that have become established in natural habitats as well. These currently make up just 5% of my present garden species. They have a high loss rate of 64%, that is, they often come and go, but those that do remain can be a nuisance, as all of them are good at spreading themselves around. The lost aliens are almost all ‘casuals’ that seldom become naturalised and often derive from bird-seed, like Phalaris canariensis (Canary-grass), Camelina sativa (Gold-of-pleasure), Panicum miliaceum (Common Millet) and the Fleabanes Erigeron canadensis and E. floribundus. One was deliberately destroyed when it appeared in 1999 – Heracleum mantegazzianum (Giant Hogweed): there are limits to a laissez-faire approach to gardening!
Of those that have become established, few are so expansive as to become a nuisance, unlike some of our natives. The only one that is a bit of a problem is Centranthus ruber (Red Valerian), mostly in its white form. Sometimes, however, these uninvited aliens can be a pleasure to have. Potentilla recta (Sulphur Cinquefoil) arrived in 2019 and seems keen to stay. It is a handsome plant. The only problem is that its chosen habitat is at the base of our up-and-over garage doors, so that it regularly gets knocked over, but resolutely returns to its erect position and seems
BSBI NEWS 152 | January 2023 15
The flora of a suburban garden and change over time
none the worse for wear. I hope it manages to seed itself into a more comfortable situation in time.
(4) Planted aliens
These plants, including garden cultivars of native species like Geranium sanguineum (Bloody Cranesbill), are normally the mainstay of a garden, but with our predilection for naturally occurring species, they only constitute 38% of our garden species. Some of them were planted before we arrived. Interestingly, they have a lower loss rate (15%) than any other group, because they are perennials and are generally designed to fare well in gardens. The ones that were lost do not seem to have any obvious reason, apart from Elodea canadensis (Canadian Pondweed) which disappeared with our pond. Lysimachia verticillaris (Whorled Loosestrife), for instance, had a period of expansion 1986–2009 and then suddenly dwindled and disappeared, perhaps as the ground became too dry.
Among the survivors, many such as shrubs remain where they are placed, but a good number (38%) are inclined to spread, some more notably than others. A few are very much inclined to take over, like Allium triquetrum (Three-cornered Garlic), whose introduction was a big mistake. It may spread by seed, but in any one year a single bulb may produce two dozen or more small bulblets, virtually impossible to remove from the soil completely when you try digging them up. One of our meadows, which has a beautiful spring display of bulb plants followed by Cowslips and Primroses, suddenly
becomes overwhelmed each summer by a mass of Three-cornered Garlic, its relative Allium roseum (Rosy Garlic) and introduced native Red Campion. Only a few Bluebell and a good number of Oxeye Daisy are left trying to hold the fort. Another thug is Pilosella aurantiaca (Fox-and-cubs), which spreads by seed and by stolons running both above and below ground.
Most shrubs are better behaved, but Prunus laurocerasus (Cherry-laurel) had been planted before we arrived. We have always tried to remove it but much still survives. At one stage its roots forced their way into our main drain and then blocked it completely. Whatever possessed the builders to plant a laurel boundary hedge beside the main drain line? There are many local woodlands where Cherry-laurel is becoming dominant and, it being of Mediterranean origin, the increasing desiccation of our woodlands will make the problem worse. Other willing spreaders include the garden form of Euphorbia amygdaloides (Wood Spurge), subsp. robbiae, Hieracium scotostictum (Spotted Hawkweed) (for which I have the late BSBI member Alan Showler to thank), Crocus tommasinianus (Early Crocus) – mostly in open grassland, but also seeding between paving stones, Hypericum calycinum (Rose-of-Sharon), Melissa officinalis (Balm) and Vinca major ‘Variegata’ (Greater Periwinkle).
Of our fifty planted aliens that survive but have not yet started spreading, 19 are bulb plants, especially cultivars of Narcissus pseudonarcissus and N. major, but also other species like N. bulbocodium The rest are a mixture of herbs like Geranium phaeum (Dusky Cranesbill) and Polygonatum × hybridum (Garden Solomon’s-seal) and typical garden shrubs like Berberis thunbergi, Fuchsia magellanica and Cotoneaster spp. Most do little to enhance the garden’s wildlife value except providing shelter and sometimes nesting sites, but I could recommend Skimmia japonica for its popularity with pollinating insects, as also is Origanum vulgare ‘Aureum’ (Marjoram). On the other hand the taxon most commonly sold as ‘Butterfly Plant’ Hylotelephium ‘Herbstfreude’, unlike the native Hylotelephium telephium (Orpine), produces no nectar at all and you do not see a butterfly anywhere near

16 BSBI NEWS 152 | January 2023
The flora of a suburban garden and change over time
Potentilla recta (Sulphur Cinquefoil) – a chance arrival in 2019
it. Mention should also be made of our Laurus nobilis (Bay) which has grown considerably in the last two years and obviously feels our climate is now sufficiently Mediterranean to start to flourish and even supports its own leaf gall-forming species, Lauritrioza alacris (Bay Sucker), a Hemipteran bug. It flowers well – will it, too, start to seed and spread?
Changes to the flora over time
No flora, no ecosystem, is constant over time, but, compared to the likes of an established chalk grassland nature reserve, a garden is particularly prone to change, receiving regular human interference and particularly subject to infusion from nearby gardens. The natural succession seen in more ‘natural’ habitats is speeded up here, with constant chance incomers, short-lived casuals, occasional planting and seeding, and a little deliberate elimination. Only the mini-meadows and hedgerow show a little more stability and something approaching an ‘ecosystem’. These are the only places where soil-growing fungi are recorded, of which 102 species have been seen over the years, indicating a more stable system. (The mushrooms Agaricus osecanus and A. urinsacens provide us with many a meal if we get to them before the slugs.) Every year the garden is different and we enjoy the surprises, while sometimes mourning the losses. It is likely that the garden is at its maximum holding capacity after 40 years, given the number of microhabitats that can be sustained, but the composition always varies.
In the detailed account above, however, a few long-term changes can be detected. Loss of habitat (the pond, well-cultivated ground) has had a major effect on variety and number of species present, but that is down to management and could potentially be changed. On the other hand, there are two processes beyond the individual’s control: nutrification from air pollution and climate warming. The effect is seen both in the garden and the wider environment.
Nutrification often occurs from poorly controlled fertiliser spray on farmland and has caused substantial losses in the hedge-bottom flora of neighbouring fields: Anthriscus sylvestris (Cow Parsley), Common Nettle and Galium aparine (Cleavers) now
often dominate this habitat at the expense of all else. Our garden fortunately avoids this effect, but airborne nitrogen and phosphorus from transport and other sources are universal, so that plants greedy for nutrients thrive and out-compete others, reducing biodiversity. Plants requiring either calcareous or acid soils particularly suffer.
Last summer (2022) has made the gradual desiccation of our soils particularly noticeable, with young Quercus robur (Pedunculate Oak) dying and many smaller plants disappearing, especially wall ferns; Alchemilla xanthochlora (Pale Lady’s-mantle) has become extinct in the area in the last couple of years, and Gentianella germanica (Chiltern Gentian) flowered this year as small wizened plants. In the garden most plants have been affected to some degree with shortened flowering periods, reduced growth and trees dropping leaves. This desiccation appears to result from a change in typical weather patterns, with summer becoming more Mediterranean in type (but mostly without the plants suited to such a climate). The drop in numbers of hoverflies and other insects has been very marked over the last 40 years, and this could have an effect on plant pollination, although bees are still regular visitors, even if one of the commonest these days is the new coloniser from warmer climes, Bombus hypnorum (Tree Bumblebee), which regularly nests under our eaves.
The change in climate affects the composition of the fauna as well as the flora, so that we may already be seeing the start of a shift in the whole ecosystem towards species of a more continental climate, with exotic species gradually replacing long-established natives. Our gardens may represent the forefront of this process – and it is in them that we can most easily study and document the changes.
Acknowledgements
My wife Val gave considerable help with the surveys (and does the brunt of the garden management!).
Tony F. Marshall ecorocker@gmail.com
BSBI NEWS 152 | January 2023 17
The flora of a suburban garden and change over time
Callitriche palustris (Narrow-fruited Water-starwort): a Cumbrian
annus mirabilis
JEREMY ROBERTS
Thisaccount of a small, elusive, intriguing, plant may I hope encourage searches in similar areas for what must be an under-recorded, and perhaps expanding, species.
Haweswater (v.c. 69, Westmorland) remains the only confirmed English location for Callitriche palustris (Narrow-fruited Water-starwort) (R.V. Lansdown, pers. comm.). It has been recorded more widely in Scotland in recent years, in v.c. 73 (Kirkcudbrightshire); several sites east of Loch Lomond in both v.cc. 86 and 99 (Stirlingshire and Dunbartonshire); and in single sites in v.cc. 87 and 89 (West and East Perthshire). In Ireland there are known sites in v.cc. H9 (Clare), H15 (S.E. Galway) and H17 (N.E. Galway). The plant is regarded as native (Stace, 2019). It has the widest global range of any Callitriche species (see Lansdown, 2008), over much of North America and Eurasia. Widespread across central and northern Europe it ‘peters out’ markedly to the west, being absent or local in the British Isles, western France and Iberia.
When Callitriche palustris was uncovered at Haweswater Reservoir in Cumbria in 2016 (Brown & Roberts, 2017) plants were scattered across an area of silt in the ‘drawdown zone’ at the head of the reservoir at an altitude of 230–240 m a.s.l. The water level on that day (in mid-July) was six vertical metres below its ‘typical high’ of 31.4 m (United Utilities, 2022). Searches in some later years found no further sign of the plant, and only small quantities of C. brutia or C. stagnalis (Intermediate and Common Water-starworts). In some years the drawdown was limited to the upper more gravelly and sandy reaches – perhaps unsuitable for germination of the plant – and in other years exposed mud was covered extensively in mats of rotting vegetation from various sources.

18 BSBI NEWS 152 | January 2023
Typical patch of fruiting Callitriche palustris (Narrowfruited Water-starwort) with Gnaphalium uliginosum (Marsh Cudweed), Lythrum portula (Water-purslane) and Riccia sp. Jeremy Roberts
By September 2022, after the notably prolonged and dry summer, the drawdown zone was very extensive (water level 12 m below ‘typical high’ and a further 6 m below its July 2016 level). The old lane- and field-walls were exposed, and very large areas of mud and silt. John Poland was in contact to say that in a short trip ‘up north’ he was hoping to have a search for Callitriche palustris, with which he was unfamiliar. Based on previous experience I cautioned John about the uncertainties of success, entirely needlessly. Phill Brown and myself met John at the head of the reservoir on 6 September. In the areas where the plant had been in 2016 there was a scatter of water-starworts, and after some casting about John called ‘here’s some black fruits!’, and the hunt was on!
The receding waterline was hundreds of metres further, and proceeding down the gently-shelving expanses of (thankfully firm) mud, normally deeply submerged, revealed abundant Callitriche palustris, colonies of mature plants with black fruits with many younger plants. A ‘guesstimate’ of the numbers was into the hundreds of thousands, and ‘a million’ was suggested!
It seems probable that the mud with the oldest, largest, plants was exposed only after the start of June. The long dry spell had then dried out these mudbanks creating extensive shrinkage fissures, visible in the photos. Many mature plants seemed rooted in the fissures, appearing as survivors of the earliest germination within the fissures during the dry period, perhaps due to better access to moisture below. With the return of damper weather, a new generation of seedlings was now apparent on the open surface of the mud, with shoots no longer than a centimetre or so. For a plant still regarded as rare in the country as a whole, the huge abundance of plants over a wide area was astounding. We now had the opportunity, and indeed luxury, to gain familiarity with this plant and its identification.
From the smaller quantities of C. brutia and C. stagnalis also present, C. palustris gave itself away at once with the abundance of fruits, many already breaking down to reveal the four black seeds inside. (On the dark mud, the tiny fruits a millimetre across could not be seen from standing. A disconcerting sight for anyone looking down from the access road would be the scatter of human forms, prostrate, or


BSBI NEWS 152 | January 2023 19
Left: a Callitriche palustris shoot, with Lythrum portula Jeremy Roberts; right: Plant being held to show scale. Trevor Lowis
Callitriche palustris (Narrow-fruited Water-starwort): a Cumbrian annus mirabilis
Callitriche palustris (Narrow-fruited Water-starwort): a Cumbrian annus mirabilis


crawling apparently aimlessly on hands-and-knees, in that huge expanse of mud! I retain evidential photographs.)
Mature plants of C. palustris consisted of a congested and strongly rooted central portion, often several intertwined. Prostrate shoots emerged to a length of 3–8 cm. Nodes towards the base of these elongate shoots indicated successful fruiting and seeds already dispersed. The middle sections showed two, three, or more nodes with ripe and ripening black fruits and apically there was every gradation from green unripe fruits to flowers. In striking distinction, the longer shoots of C. brutia
plants showed no sign whatever of flowers or fruits developing on their outer portions, and the very few fruits observed were on the basal portions, often tucked down into the mud from the semiimmersed stems. (Fruits were more-or-less sessile, and the assumption is that these brutia plants are of subsp. hamulata, the typical Callitriche of Lake District waterbodies, distinctive enough when in its submerged form; subsp. brutia, distinguished by often longer peduncles, has yet to be recorded in Cumbria.)
Fruits provide immediate distinctions between C. palustris and other British and Irish species. The colour of the fruit is often given as a crucial feature – e.g. Stace (2019), ‘The black fruits (when fully ripe) are diagnostic’ – but note that Lansdown (2008, p. 22) lists C. brutia and some non-British species as having ‘black fruit’ when ripe. Two further features of the fruit are very useful: (i) its narrow dimensions (width slightly narrower than length) and somewhat ‘heart-shaped’ appearance from the side, and (ii) the almost complete lack of visible styles. Very short arching styles can rarely be seen on the very closest inspection, but generally are lacking. The other species at this site have long and more persistent styles, in C. stagnalis variously erect, arching or spreading, and in C. brutia strongly reflexed downwards, hugging the sides of the fruit (indeed often emerging sideways from the upper portion of fruits, with rows of cells above the point of emergence).
The jet-black colour of the fruits is in reality the colour of the four seeds within. To follow Lansdown’s (2008) explanation, the fruit is a schizocarp, and has a thin and translucent outer coat, which disintegrates with the maturation of the four mericarps containing the seeds. It is the rim of the mericarp which forms the translucent so-called ‘wing’, which provides significant characters. In C. palustris a wing is wellmarked around the apical half of the mericarp, but absent towards the base (in our form). In brutia and stagnalis, the wing is present right around the structure.
A very obvious feature of C. palustris at Haweswater was that the long fruiting stems had sparse, or no,
20 BSBI NEWS 152 | January 2023
Top: Callitriche palustris shoot with ripe and unripe fruits; bottom: distinctive black appearance of the fruits due to the dark seeds showing through the translucent mericarp. Jeremy Roberts
development of nodal roots along their length, so that – very handily – a shoot could be grasped by its tip and then lifted away almost to its base. This very ‘open’, spreading and loose growth of C. palustris was in marked contrast to the dense and mat-forming patches of C. brutia, clinging firmly to the mud. C. brutia nodal root development was far greater: pulling at a shoot tip merely caused the stem to fracture before the first node down, and so on downwards. To extricate a shoot of C. brutia intact required a blade below to sever the roots! At least at this site and in this season the vigorous fruiting on all longer shoots in C. palustris, compared to the sparse fruiting but strong vegetative mat-forming growth in C. brutia, suggest contrasting annual, versus perennial, strategies.
Leaves of C. palustris towards the base of shoots were lingulate, linear or narrowly expanded, and often distinctively deflexed, whilst more apical internodes had leaves slightly broader. No truly spathulate leaves were observed. The fresh apical growth of mature plants was a mid-green colour, but at this late stage of the season many leaves and some whole stems were ageing to yellow, making the plants very conspicuous. C. brutia had leaves of similar shapes, but remaining a deep green throughout.
Continuing down towards the water-line revealed smaller and less-developed plants. However, beyond the last plants of this and other species there was some hundreds of metres of recently-exposed bare mud, supporting the notion that this generation of plants resulted from seeds germinating after retreat of the water, i.e. ‘terrestrially’, and not from plants initially developing underwater and only exposed with water retreat.
The huge numbers of fruit on offer suggested collection of seed for the Millennium Seed Bank (MSB). Cloth bags were sent by them, and permission from owners United Utilities obtained, and on 16 September a crack team assembled, and Phill, Trevor Lowis, Gary Lawrence and myself spent an entertaining couple of hours collecting shoots. Each shoot required scrutiny with pocket lens to ensure its identity. After partial drying on tissues overnight prior to posting, it was reassuring to see
how very many seeds were already shed. The final total is likely to be into the thousands. We await feedback from MSB as to how the collection fared in curation, but the haul will be satisfyingly above the ‘147’ seeds sent in 2016!
Using various sources, the area of mud supporting Callitriche palustris was estimated to be at least 150 × 270 metres, or 40,000+ square metres. Over the whole site, an average of only 25 plants per square metre would thus provide a million plants. Substantial plants in some typical areas were counted in a random sample of five one-squaremetre quadrats, to suggest a population density in the region of 100–200 per square metre. (The photograph below of a particularly dense patch covers close to a third of a square metre of habitat. Using the software ImageJ to help count the plants of all sizes in the image revealed roughly 120 – a density of c. 350 plants per square metre!)
A further visit by the author on 26 September allowed time for investigation into the plant communities. The main colonies of Callitriche palustris were in communities seeming to match closely the National Vegetation Classification OV31, the ‘Rorippa palustris-Filaginella uliginosa community’ of Rodwell (2000) – with the Rorippa being here represented by R. islandica (Northern Yellow-cress) in quantity, and Filaginella (Gnaphalium) uliginosum

BSBI NEWS 152 | January 2023 21
Dense patch of c. 120 plants of Callitriche palustris with OV31 associates. Jeremy Roberts
Callitriche palustris (Narrow-fruited Water-starwort): a Cumbrian annus mirabilis
(Marsh Cudweed) in overwhelming abundance, with small but already-fruiting plants. Other associates typical of this association and abundant were the knotweeds Polygonum depressum (= P. arenastrum; Equalleaved Knotgrass), Persicaria hydropiper (Water-pepper) and P. maculosa (Redshank). The much more local P. minor (Small Water-pepper) was another frequent associate. Lythrum portula (Water-purslane) was codominant in many areas and most attractive in its vinous flowers and flushing. Rodwell adds that ‘this community can provide a locus for the nationally rare Limosella aquatica’ – the delightful Mudwort, which was indeed present, but scattered and hardly yet flowering at that date. Juncus bufonius (Toad Rush) and Poa annua (Annual Meadow-grass) were other species of the OV31 community present in quantity.
It is reassuring to know that Callitriche palustris is well established in the muddy seed bank at Haweswater and may emerge and prosper in future years – when conditions suit. Richard Lansdown remarked (pers. comm.), ‘… we will never entirely

understand and certainly never be able to predict the fluctuations in populations of mud plants; species such as Callitriche palustris and (particularly) Limosella aquatica show massive population fluctuations, often with only a few hints as to why’. Rodwell (2000, p. 432) comments in similar vein, ‘Typically, like the commoner species in this community [OV31], populations of Limosella can vary greatly in size from year to year in any one place. The characteristic habitats here are not only unstable but quite often not precisely congenial for colonisation by a particular species’.
Acknowledgements
I am grateful to John Poland for initiating the search in the first place; John, Phill Brown, Gary Lawrence and Trevor Lowis for sharing the tasks, and offering the sage comments that I anticipate from these gentlemen in discussions on the day and over an earlier draft; Richard Lansdown, referee for Callitriche, for several exchanges over the find and
22 BSBI NEWS 152 | January 2023
Callitriche palustris (Narrow-fruited Water-starwort): a Cumbrian annus mirabilis
Seed collectors Phill, Jeremy and Gary, and the Callitriche habitat. Trevor Lowis
Two early records of the rare Rumex rupestris (Shore Dock)
the draft of this article; and John Gorst, ecologist at United Utilities, for permissions and other support.
References
Brown, P.L. & Roberts, F.J. 2017. Callitriche palustris (Narrowfruited Water-starwort) in Westmorland (v.c. 69), new to England. BSBI News 135: 38–39.
Lansdown, R.V. 2008. B.S.B.I. Handbook No. 11: Waterstarworts (Callitriche) of Europe. Botanical Society of the Britain Isles, London.
Rodwell, J.S. (ed) 2000. British Plant Communities, Vol. 5: Maritime Communities and Vegetation of Open Habitats. Cambridge University Press, Cambridge.
Stace, C.A. 2019. New Flora of the British Isles (4th edn). C & M Floristics, Middlewood Green, Suffolk.
United Utilities: website ‘Haweswater Reservoir Monitoring Station’: riverlevels.uk/haweswater-reservoir-bampton#google_ vignette (accessed 6 October 2022).
Jeremy Roberts
Eden Croft, 2 Wetheral Pasture, Carlisle, CA4 8HU fjr@edencroft2.co.uk
DAVID PEARMAN
Someyears ago I came across a paper by the then Curator of the Herbarium in Edinburgh, Mr F.M. Webb (Webb, 1879). In this note he wrote: ‘Rumex rupestris . For an account of this as a British plant see Journal of Botany xiii, pp. 294, 337, and xiv, p.1, with plate. It was first noticed in Jersey by Rev. W.W. Newbould in 1841 [seemingly an error for 1842], but passed almost out of sight until studied and determined by Mr Briggs to be a plant of the Plymouth coast in 1875. I recognised in our collections two sheets each with one good specimen of the plant gathered by Professor Balfour at Babbacombe, 26th Aug. 1839 (one is marked “near Exeter, 1839”, that being his headquarters).’
I was finally able to visit the Herbarium in March 2022, and found the two sheets. They had good fruit, and I was sure that they were this species. I showed the scans to colleagues in Cornwall and Devon, Ian Bennallick and Andy Byfield, and they concurred. The characteristic large tubercles on all three tepals are clearly visible. Professor J.H.
Balfour
Rumex rupestris (Shore Dock) in typical habitat at Molunan, Mevagissey, E. Cornwall (v.c. 2), September 2017. Ian Bennallick

BSBI NEWS 152 | January 2023 23
Two early records of the rare Rumex rupestris (Shore Dock)
(1808–1884) was Regius Keeper of the Herbarium at Edinburgh, the Queen’s Botanist at Edinburgh from 1845–1879 and founded the Botanical Society of Edinburgh in 1836.
These specimens are very interesting because the earliest record previously known for England, was 1859, from Three Cliffs Bay, Glamorgan; it also predates the discovery in Jersey in 1842 (Pearman, 2017) which itself was not clarified until 1876 (Trimen, 1876). Nineteenth century botanists
struggled in identifying this species, and to be fair, many current botanists do too.
However, there is a further conundrum. There are old records from east of Exeter, from Lyme Regis and Ringstead in Dorset, though personally I have reservations about some of those, and none have persisted. Other than these the furthest east that R. rupestris has ever been recorded in Britain is from Slapton Ley, about 18 miles to the west of Babbacombe. Initially I worried about this, but bearing in mind the above-mentioned difficulties of identification, I do not consider this a problem. I assume that there would have been plenty of suitable habitat around Babbacombe at that time.
This is yet another justification for the retention and use of herbarium specimens.

References
Pearman, D. 2017. The Discovery of the Native Flora of Britain & Ireland. Botanical Society of Britain & Ireland, Bristol.
Trimen, H. 1877. Rumex rupestris Le Gall. as a British plant. Journal of Botany 14: 1–4.
Webb, F.M. 1879. Notes upon some plants in the British Herbarium at the Royal Botanic Garden, Edinburgh. Transactions and Proceedings of the Botanical Society of Edinburgh 13: 88–114.
David Pearman
Algiers, Feock, Truro TR3 6RA
dpearman4@gmail.com
24 BSBI NEWS 152 | January 2023
The specimen of Rumex rupestris (Shore Dock) in the Edinburgh Herbarium. The label reads ‘Ex Herb. T. H. Balfour, Rumex rupestris, LeGall. Babbicombe [sic] Aug. 26. 1839’
Two early records of the rare Rumex rupestris (Shore Dock)
MICHAEL BRAITHWAITE
History
Pyrola minor (Common Wintergreen) was first reported in Berwickshire in Dr George Johnston’s Flora of 1831 where five localities were listed. It was recorded assiduously over the remainder of the nineteenth century, being among the most soughtafter species along with the scarcer orchids. Botanical recording was at a low ebb for the first half of the twentieth century, but has since achieved almost complete coverage of habitats likely to hold Pyrola
I first recorded Pyrola in Berwickshire in 1979, and thus have 40 years’ experience of the species. After some years’ experience of searching for historical localities, along with more general fieldwork, it became clear that the majority of the nineteenthcentury localities had been lost but that I was finding new localities, often in places where nineteenthcentury records could have been expected in view of the other early records from the localities in question.

Few of the nineteenth-century records were made in ancient woodland, reflecting the rarity of ancient woodland in the vice-county. Most were made in plantations and avenues where much of the planting dated from 1780 to 1820. There had clearly been active colonisation of Pyrola in the early part of the nineteenth century.
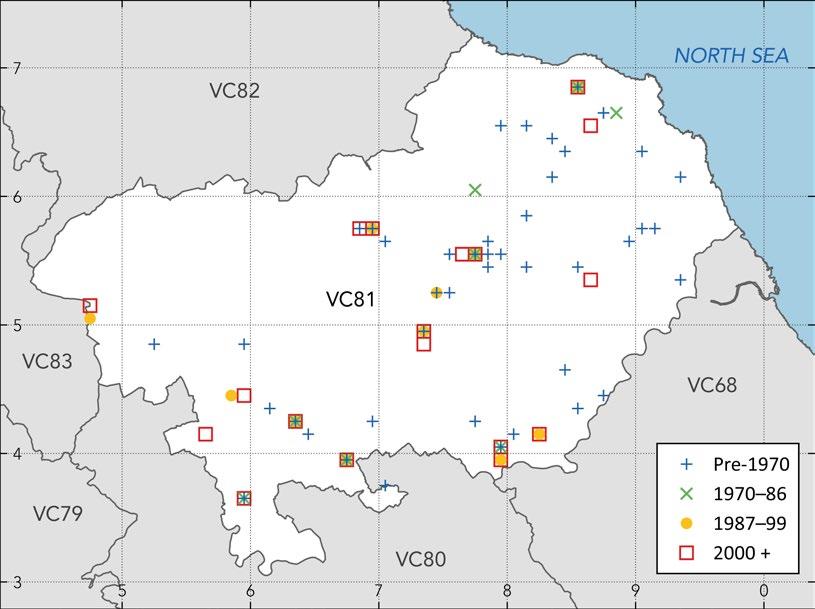
A number of my records, and those of other contemporary recorders, were made in mosses (mires). No nineteenth-century records were made in mosses, though one from ‘[moorland] near Dowlaw Dean’ probably relates to Long Moss, Coldingham Common NT86, where there had been extensive peat cutting followed by colonisation by birch and willow and where Pyrola still grows. Records are mapped in Figure 1.
BSBI NEWS 152 | January 2023 25
Pyrola minor (Common Wintergreen), Green Ride, Marchmont (NT7348), on a bank under Fagus sylvatica (Beech), 15 June 2021. Robin Cowe
Modelling the history of Pyrola minor (Common Wintergreen) over 200 years in Berwickshire (v.c. 81)
Modelling the history of Pyrola minor (Common Wintergreen) over 200 years in Berwickshire (v.c. 81)
26 BSBI NEWS 152 | January 2023
Figure 1. Map showing the monad distribution of Pyrola minor in Berwickshire (v.c. 81)
0 5 10 15 20 25 30 1830 1850 1870 1890 1910 1930 1950 1970 1990 2010 Populations Ancient woodland
Figure 2. Graph showing the change over time in habitat preference of Pyrola minor in Berwickshire (v.c. 81)
Mosses Plantations Scrub
Habitat
Pyrola minor is found in slightly moist mossy ground under relatively mature trees. The most favoured tree species are Fagus sylvatica (Beech) and Betula (birch), but the shade of Pinus (pine), Quercus (oak) and Salix (willow) are also colonised. The series of fine ancient oakwoods around Abbey St Bathans (NT76) have but a single poorly-localised record from 1970, which has not been refound. The colonies in mosses favour carr woodland, colonising over areas of the moss where there has been peat cutting or partial drainage. The woodland expansion causes further drying out. This succession is a feature of many of the Berwickshire mosses. Occasionally, colonies are found in scrub on broad roadside banks.
• Populations counted at 1 km scale. Where a colony marginally overlaps a 1 km boundary, the overlap is ignored.
• Ancient woodland populations are assumed to have arisen in the indefinite past and to have been lost 20 years after the last record.
• Populations in mosses are assumed to have colonised 20 years before the first record and to have continued indefinitely.

• Populations in plantations and avenues are assumed to have colonised 20 years before the first record and to have been lost 20 years after the last record. This implies a 60-year cycle, being the life of a typical colony before it is lost to clear-felling.
• Populations in scrub are treated the same as those in plantations. Populations have been counted for each 20-year period between 1820 and 2019. The period 1820–1839 is identified as 1830, and so on.
Results
The graph (Figure 2) highlights the change in habitat mix outlined above. Notably, the total number of populations at 1 km scale has remained fairly constant over 200 years, if some allowance is made for the uneven history of recording. Losses have been broadly compensated for by the colonisation of mosses and recently planted woodland. If the model used can be accepted as valid, then the ability of wind-dispersal to enable Pyrola to exploit newlyavailable habitat is amply demonstrated.
References
Dispersal
The tiny seeds of Pyrola are wind-dispersed. Most of the dispersal is over a few hundred metres in a wood, resulting in a few daughter colonies. Long-distance dispersal is much rarer, but this study demonstrates that it does occur over distances of 10 km or more.
Modelling the history
I have attempted to model the history of Pyrola minor in Berwickshire using the following parameters:
Braithwaite, M.E. 2013. Berwickshire BSBI Botanical Site Register, privately circulated, Hawick.
Braithwaite, M.E. 2014. A Short Flora of Berwickshire, privately circulated, Hawick.
Braithwaite, M.E. & Cowe, R. 2019. Berwickshire BSBI Botanical Site Register update 2019, privately circulated, Hawick.
Johnston, G. 1829, 1831. A Flora of Berwick-upon-Tweed, J. Carfrae & Son, Edinburgh and Longman, London.
Michael Braithwaite
Clarilaw Farmhouse, Hawick, TD9 8PT mebraithwaite@btinternet.com
BSBI NEWS 152 | January 2023 27
Redpath Moss NT5936, moss carpet under birch, 8 July 2010. Michael Braithwaite
Modelling
the history of Pyrola minor (Common Wintergreen) over 200 years in Berwickshire (v.c. 81)
Ivy confusions?
Ivyspecies are not sprinkled over the landscape like chickenpox spots on a human body! Only Common Ivy (Hedera helix) and Irish Ivy (Hedera hibernica ‘Hibernica’) occur near each other. The first is native for much of the UK mainland, while Irish Ivy is commonly cultivated and frequently naturalised. By contrast the native Atlantic Ivy (Hedera hibernica) is found throughout Ireland and along a narrow strip of western Britain, from South Ayrshire down to Devon and Cornwall where it is the sole Ivy. Irish Ivy and Atlantic Ivy occur on different sides of the country. Atlantic Ivy is on the Atlantic seaboard, and Irish Ivy is found in the centre and east. Only in two instances has a colony of Irish Ivy been seen in Atlantic Ivy country since research began in the early 1970s!
The map for Atlantic Ivy (as Hedera helix subsp. hibernica) in the New Atlas (Preston et al., 2002) shows a good distribution of Irish Ivy (H. ‘Hibernica’) as red dots (i.e. non-native status) while the blue dots (native) are reasonable for Atlantic Ivy (H. hibernica). It is shown as much denser for Northern Ireland in McAllister & Marshall (2017).
Ivy identification
Leaves
Irish Ivy (H. hibernica ‘Hibernica’) is an easy plant to spot. It passes directly from seedling leaves to the sub-fertile stage of foliage. It has uniform, large, glossy leaves, with short, broad lobes. The leaves do not open flat, so the light catches one half of each leaf. This Ivy can form cascades down cliffs or walls, or sheets over the ground – where this feature is eye-catching. Unlike Common Ivy foliage, it seldom bronzes in cold and Irish Ivy leaves are a fresh emerald.
Common Ivy (H. helix) passes through many development phases from seedling to flowering foliage. The leaves are often dull until the fertile stage. They are often dark green, bronzing in cold, even turning black. Much smaller than those of Irish Ivy and most Atlantic Ivy, they become larger just before the flowering stage and are leathery in texture.
Atlantic Ivy (H. hibernica) is, on the whole, much larger in its leaves than Common Ivy but, if stressed by cutting winds (as at Cairnryan near Stranraer)

28 BSBI NEWS 152 | January 2023
ALISON RUTHERFORD Atlantic Ivy (Hedera hibernica). Michael Jeeves
it can have H. helix sized foliage. It is probably as variable in leaf forms as Common Ivy.
Scent
The odour of cut or bruised twigs or leaves of Common Ivy is found to be acrid and unpleasant to many people, while Atlantic Ivy is sweet, aromatic and resinous. Irish Ivy has a less strong odour, possibly because it’s at the nearly fertile phase when many characters such as running, climbing and phase-changing diminish.
Habitats
Common Ivy enjoys mortared walls, but does poorly on acid soils. Atlantic Ivy, on the other hand, is happy on acid soils and can be found among granite boulders, Bracken (Pteridium aquilinum) and Gorse (Ulex europaeus). Irish Ivy is frequently grown and widely naturalised. It is found in hedges, woodlands and garden dumping spots. It is not fussy about soils, but not found at much of an altitude.
Looking at the hairs
In Common Ivy, the hairs are like a sea anemone – a little stalk with the rays like tentacles bristling in all directions. In very new leaves, when the new foliage at the shoot tips have barely drawn apart, these hairs mesh together, giving a bristling, fuzzy appearance. In both Irish Ivy and Atlantic Ivy the hairs lie flat on the leaf surface, like starfish on the seabed, and the rays look like lengths of spider’s web (Figure 1 & 2).
To check the hairs an 8× or 15× lens may be easier than a 20×, but try different ones and different degrees of light. It is not necessary to count the rays of the hairs to distinguish Common Ivy from Irish or Atlantic Ivy. Even with the naked eye (or in a close-up photo as in Figure 2) it is possible to discern whether the hairs are raised away from the surface or lying flat against it.


Collecting Ivy to identify
Ivy specimens, even from hard-grown, windswept or damaged plants, will produce new growth with perfect hairs. If the colony is frosted or frozen and

this is the only chance of reaching that area, put the cuttings in cold water for half an hour before preparing them.
Collect juvenile fat, whippy shoots and sprinkle with water before placing in a plastic bag (Ivy dislikes being dried out). Multiple samples can go in the same bag if labelled. Put the shoot into a jar and place in a large freezer bag, blow into it to inflate and secure
BSBI NEWS 152 | January 2023 29
Ivy confusions?
Figure 1. Microscope photos of the stellate hairs of Common Ivy (left) and Irish Ivy (right). Courtesy Liverpool University (from McAllister & Rutherford, 1990).
Figure 2. The hairs of Common Ivy (top) can clearly be seen rising from the surface, making it look ‘hairy’. The hairs of Atlantic Ivy and Irish Ivy (bottom) are flattened. Michael Philip
Atlantic Ivy (Hedera hibernica) clothing the rocky coastline at Port Castle Bay, Wigtownshire.
Michael Jeeves
with a ‘twistie’. Set the jar on a window sill: in high summer, a south-facing window may be too fierce. Conventional rooting also works – in a pot of compost inside a freezer bag. Cut the stalk in 6–12 cm lengths, remove any leaves that would be buried and gently rub off the tiny bud (or you’ll get a leaf instead of roots). Use 50-50 John Innes No.2 (soil-based) and multipurpose compost – or topsoil if John Innes is not available. Protect with a freezer bag as described above and set in good light. Rooting varies with the time of year and the temperature, but with the jar or pot of cuttings you will have perfect hairs.
Ivy research
Seeing Irish Ivy in a groundcover book in the 1970s, and realising it was locally more abundant than Hedera helix, I wondered what was its origin? Hoping to discover clues, Chris Wilcock of Aberdeen University began counting chromosomes of different species till the lab was almost buried in greenery and I feared to ask about progress! Allan Stirling, then Vice-County Recorder for v.c. 99, suggested Hugh McAllister might be able to help. Luckily he said yes: Liverpool University had glasshouses and ground, so he was able to accommodate ivies from all countries, strike cuttings, count chromosomes and plant them out.
The BSBI Irish Ivy Survey (1975–79) brought a flood of samples. In 1990 Hugh and I had a paper on Irish, Atlantic and Common ivies (McAllister & Rutherford, 1990). Hugh and Rosalyn Marshall published a monograph in 2017 in the Royal Horticultural series (McAllister & Marshall, 2017). It lists 15 double-column pages of references, but few people outside Hedera fanciers seem to be aware of the research of the last 10 to 15 years.
Hopefully this brief article will inspire greater confidence, especially in the identification of Common Ivy. Once a plant has been identified as Hedera helix, we can allow vague terms such as Hedera helix agg. or Hedera helix s.l. to become obsolete. At a later date it is hoped to produce a similar account of the alien ivies.

References
McAllister, H. & Marshall, R. 2017. Hedera: The Complete Guide. Royal Horticultural Society, Peterborough. McAllister, H.A. & Rutherford, A. 1990. Hedera helix L. and H. hibernica (Kirchner) Bean (Araliaceae) in the British Isles, Watsonia 18(1): 17–15.
Preston, C.D., Pearman, D.A. & Dines, T.D. (eds) 2002. New Atlas of the British and Irish Flora. Oxford University Press, Oxford.
Rutherford, A. 1979. The BSBI Irish Ivy Survey, BSBI News 22: 8–9.
Alison Rutherford
19 South King Street, Helensburgh, G84 7DU
30 BSBI NEWS 152 | January 2023
Ivy confusions?
Caltha palustris var. radicans: overlooked and underrecorded?
RICHARD LANSDOWN & TIM PANKHURST
During a visit to explore the bryophyte flora of some Norfolk fens in early 2022, the authors found, at Smallburgh Fen (TG327245), flowering plants of Caltha palustris (Marsh-marigold) with smaller plantlets developing on creeping stems attached to the main plant (Plate 1; all photographs taken by RVL). Each plant had one, or occasionally two, flowers. These plants could clearly be assigned to C. palustris var. radicans (T.F. Forst.) Beck (1886), a variety hitherto unrecorded in Norfolk. The plants found at Smallburgh were in high quality calcareous mire allied to the M13: Schoenus nigricans mire (Rodwell, 1991).
With hindsight, the authors felt they had seen similar plants elsewhere in East Anglian fens but had not identified them. RVL subsequently found populations of C. palustris in Scotland along the margin of the River Spey which also could be assigned to var. radicans (Plate 2). The limited number of recent records of this variety, together with the previously unknown habitat in the UK suggested that var. radicans might be more widespread than generally thought and it seems worth providing some information here to inform recording.
In 2020, RVL became involved in a project to prepare a guide to the identification of aquatic plants of north-west Europe (Schou et al., in press). This provided an opportunity to understand aspects of the identification and distribution of aquatic plant taxa from the perspective of botanists from continental Europe and led to a re-appraisal of many taxa, including the discovery of two new species for the UK: Wolffia columbiana and W. globosa (Lansdown et al., 2022), it also raised his awareness of some other taxa that are less well studied in the UK.
Caltha palustris is a highly variable species and taxonomic studies have generally concluded that most variation in the species is environmentally
induced; however there is general consensus (apart from Plants of the World Online [Kew.org, 2022] which assigns it to synonymy within C. palustris var. palustris) that plants which have stems rooting at the nodes (Plate 3) should be recognised as separate, either as a variety (e.g. Smit, 1971; Woodell & Kootin-Sanwu, 1971), a subspecies (e.g. Chrtková & Jarolimová, 1999; Cieślak, 2004) or even a species in its own right (e.g. Praeger, 1934). Praeger (1934) provides a detailed description of his C. radicans However, his C. radicans clearly differs from the current understanding of C. palustris var. radicans and so descriptive and habitat details cannot be directly compared. Most authors agree that the most consistent character separating C. palustris var. radicans from var. palustris is that the stems root at the nodes (Woodell & Kootin-Sanwu, 1971; Cieślak, 2004). Schou et al. (in press) provide further characterisations, including that var. radicans often forms large stands due to the prostrate, rooting stems and production of daughter plants, as well as having smaller, paler yellow flowers which are usually solitary (Plate 4). In contrast, var. palustris usually produces clusters of large flowers, typically held clear of the foliage (Plate 5) on plants forming discrete clumps (Plate 6), although some non-native cultivars can have flowers held lower, among foliage which tends to be much larger (Plate 7), and other cultivars differ further still (Plate 8).
C. palustris var. radicans is a taxon which, in the UK, is typically considered to be restricted to ‘mountain flushes and lake shores in N. & W. Britain and Ireland’ (Preston et al., 2002). Its presence in calcareous fens and on the margins of a large lowland river is therefore noteworthy. However, in continental Europe, it is reported from open and wooded swamps, the margins of ponds, lakes, ditches, streams and flushes, as well as springs and
BSBI NEWS 152 | January 2023 31
Caltha palustris var. radicans: overlooked and under-recorded?
spring-fed meadows, typically on open, moist to wet ground with oligotrophic to mesotrophic soil and water (Schou et al., in press).

C. palustris var. radicans is considered to have a more northern distribution than the nominate variety and, in Europe, is mainly found in Great Britain, Denmark, Norway, Sweden and Finland (Schou et al. in press); however it has also been found in central Europe, i.e. in Poland, albeit in the north of the country (Cieślak, 2004). It has been reported from throughout the UK; however, the only post2000 record from England is from the Isle of Man and most recent records are clustered around the central highlands of Scotland (BSBI maps). It is
definitely worth inspecting plants of C. palustris more closely, particularly small, few-flowered plants in fens, and it would be interesting to revisit the sites of the old records on the BSBI database.


References
Chrtková, A. & Jarolimová, V. 1999. Cytotaxonomical study of Caltha palustris Preslia 71: 349–360.
Cieślak, E. 2004 Morphological variability of the Caltha palustris L. complex (Ranunculaceae) in Poland. Acta Societatis Botanicrum Poloniae 73: 193–201.

Lansdown, R.V., Kitchener, G. & Jones, E. 2022. Wolffia columbiana and W. globosa (Araceae) new to Britain. British & Irish Botany 4(1): 14–26.
Kew.org 2022. Plants of the World Online: powo.science.kew. org (accessed 10 November 2022).
32 BSBI NEWS 152 | January 2023
Plate 1. Caltha palustris var. radicans (Marshmarigold) Smallburgh Fen, Norfolk, April 2022.
Plate 2. Caltha palustris var. radicans, Mains of Cromdale, Moray, August 2022.
Plate 3. C. palustris var. radicans showing the procumbent stem, Meall Gorm, East Perth, June 2010.
Plate 4. Caltha palustris var. radicans following the line of a small flush at Meall Gorm and showing sparse flowers, East Perth, June 2010.
Caltha palustris var. radicans: overlooked and under-recorded?
Preston, C.D., Pearman, D.A. & Dines, T.D. (eds) 2002. New Atlas of the British and Irish Flora. Oxford University Press, Oxford.
Rodwell, J.S. (ed) 1991. British Plant Communities, Volume 2: Mires and Heaths. 128–139. Cambridge University Press, Cambridge.
Schou, J.C., Moeslund, B., van de Weyer, K., Wiegleb, G., Lansdown, R.V., Holm, P., Baastrup-Spohr, L. & SandJensen, K. in press. Aquatic plants of North-west Europe. Princeton University Press, Princeton, New Jersey.
Smit, P.G. 1968. Taxonomical and ecological studies in Caltha palustris L. II. Mededeelingen van het Botanisch Museum en Herbarium van de Rijks Universiteit te Utrecht 300: 280–292.
Woodell, S.R.J. & Kootin-Sanwu, M. 1971. Intraspecific variation in Caltha palustris. New Phytologist 70: 173–186.



Praeger, R.L. 1934. Caltha radicans in Ireland. Irish Naturalists’ Journal 5(5): 98–102.

Richard V. Lansdown
45 The Bridle, Stroud, GL5 4SQ rvlansdown@gmail.com
Tim J. Pankhurst
Plantlife, Brewery House, 36 Milford St, Salisbury, SP1 2AP
Tim.Pankhurst@plantlife.org.uk.
BSBI NEWS 152 | January 2023 33
Plate 5. Caltha palustris var. palustris showing the clusters of flowers held high above the foliage, Quarry Bank, Syall, Cheshire, April 2022.
Plate 6. Caltha palustris var. palustris forming a discrete clump, Stroudwater Canal near Stonehouse, Gloucestershire, April 2011.
Plate 7. Caltha palustris, unnamed cultivar, Frampton-on-Severn, Gloucestershire, April 2010.
Plate 8. Caltha palustris ‘flora pleno’ Slimbridge WWT reserve, Gloucestershire, April 2003.
and under-recorded?
Caltha palustris var. radicans: overlooked
Take the risk – do a FISC!
SARAH WHILD & SUE DANCEY
Pictureforty botanists of various abilities arriving at Preston Montford Field Centre in a sunny week in 2005, not knowing what would be facing them. We had devised a wide range of botanical trials and tribulations for these plucky volunteers. They were told to bring some identification guides and a hand lens – other than that there were no certainties – apart from there being cake, and plenty of it.
Field Identification Skills Certificates started in 2005 when we were given a small research grant by BSBI to see if it was possible to devise a botanical ‘driving test’ – a way of awarding a botanist with a ‘certificate of competence’ – but most importantly, stating on the certificate just how good that botanist was at field identification. We both had plenty of experience in setting and marking botanical identification tests as part of academic assessments –but this tested the learning process rather than how good the student was if parachuted into a botanical site and left to survey it.
We had published a general field skills pyramid at the British Ecological Society conference on the decline in field skills in 2002, so devising a botanical skills pyramid based on this was straightforward. The grant allowed us to advertise for both volunteer botanists and complete beginners – we needed a wide range of (subjective) skill levels. Initially we used two different lab tests. The first – ten species that were relatively widespread and straightforward to identify – were identified without any additional aids such as identification guides. Essentially you either knew the plant or you didn’t. The next twenty species could include national rarities, hybrids, common species, difficult species, and plants from a really wide range of habitats throughout Britain (for example montane or saltmarsh species). These could be identified with any identification guides available, and a microscope was available for participants to use if they wished.
During the FISC trials, we set out a two by two metre quadrat for participants to record – a small ‘field’ area, where they recorded against a ‘gold standard’ botanist recording at the same time. There was a test including five common trees on site and three aquatic species that the participants had to grapnel out of a pond. The brave volunteers certainly earned their cake and lunch. We analysed the results using Excel, initially to see if the marks fell into bands for each of the tests – essentially, which of the tests sorted botanists into some sort of ladder of ability. The tree identification, aquatic plants, and the quadrat test were dropped, as none of these distinguished one botanist from another. The lab tests certainly did sort botanists into ability bands, or levels, and so did the field test; but after refining the field test sufficiently, we realised, with help from Quentin Groom, that false positives were a really important part of how good a botanist is.
What are botanical false positives? Well, in a nutshell, recording what isn’t there – the field test element involved recording against a ‘good’ botanist recording at the same time, under the same conditions, and the score would be a percentage of the gold standard’s total, so there was an incentive to write down as many species as one could, in the hope some of them might be correct! There were also fabulous made-up names too, christened Jabberwockies by Quentin: Burweed, Separated Rush, Carex glauca and Silene jacobaea are some of the weird and wonderful or just desperate names that came out in some of the field tests (where all participants can use field guides, so no excuse really!).
Participants were awarded one point for each correct species that occurred on the gold standard list – or was at least very likely to be there if the gold standard recorder had missed it. If they recorded cautiously, for example Carex sp., then half a mark would be awarded (but only once, irrespective of how many sedge species were present).
34 BSBI NEWS 152 | January 2023
Take the risk – do a FISC!


BSBI NEWS 152 | January 2023 35 Take the risk – do a FISC!
FISC participants undergoing field tests at Wigan, August 2022. Josh Styles
FISC lab-based identification test at Wigan. Josh Styles
As a disincentive to record just anything, and to encourage cautious recording, the false positives were added up, and a score given to reflect how many were recorded. This part of the FISC results ended up being one of the key components for identifying good recorders. Our final scoring method gave equal weight to lab test 1, lab test 2, field survey and false positives (if you are interested in the marking scheme, all of the FISC protocols are openly available on the BSBI website (bsbi.org/field-skills).
In addition, we got participants to estimate their skill level on the botanical skills pyramid before the tests – it was amazing to see how good botanists were at assessing their own level (certainly for Level 3 and above). Lower skilled botanists tended to overestimate their ability.
Thus we had our marking scheme, and what seemed like a reasonably robust method, so we launched the first full FISC at Preston Montford Field Centre in 2007. Amazingly enough, around twenty people signed up to pay a modest amount for a day of botanical trial and torture! The following year we ran three FISCs in Shropshire, then after that, they really took off. I think when we were devising them initially, we thought local volunteer botanists would be the target audience, but consultants dominate FISC applicants now. We were delighted to work with Sally Haynes from CIEEM so that FISCs were incorporated into their skill standards, and Natural England embraced FISCs fully, and run several FISCs each year for their botanists, and also for the odd external applicant.
In 2017 we held a major review of FISCs and invited academics, consultants and botanical experts to take part and review the protocols and marking structure. It was good to note that although there were some changes, and plenty of clarifications as a result of the review, the marking scheme has pretty much stood the test of time.
This year, we have been delighted to be involved in BSBI’s interim FISC working group, chaired by Julia Hanmer, and we have worked with Dr Mary Dean and Anthony Thomas to produce a watertight
set of documents to support FISC providers, and make sure the awards are as robust as possible. We’ve also developed materials to train up new FISC assessors, so BSBI can widen overall FISC provision. Bringing on board new FISC providers and assessors will take training and time, but it is heading in the right direction and attracting lots of interest. As we are finishing this article, we are signing a deed, handing all intellectual property rights to BSBI, so that FISCs continue to be a BSBI award. More than a thousand botanists have taken a FISC, and in the next BSBI News there will be dates for 2023 FISCs and information about how BSBI are working to increase provision in future years. Go on – take a risk – do a FISC!
Acknowledgements
We are enormously grateful to all the fabulous botanical and educational folk who have helped us along the way. Thanks to the Field Studies Council, Preston Montford Field Centre, Mark Duffell, Jenny Duffell, Alex Lockton, Quentin Groom, Richard Gornall, John Warren, Toby Hart (who did a FISC at every centre to compare them!), Nick Law, Val Gateley, Josh Styles, Mary Dean, Anthony Thomas, the team from Natural England including Andrea Perkins, Alison Jukes, Andy McLay, Alex Prendergast, Rob Large, John Martin and Steve Parker, and the Kent Wildlife Trust team including Jill Evington and Ros Bennett. The Training and Education Committee (now the Skills and Training Committee) supported the creation of FISCs. I’m sure we’ve missed lots of people out – sorry – but thank you!
Sarah Whild
sarah.whild@bsbi.org
Sue Dancey
thatched1960@gmail.com
For further details see the BSBI website: bsbi.org/ field-skills
36 BSBI NEWS 152 | January 2023
Take the risk – do a FISC!
BEGINNER’S CORNER
Making sense of mouse-ears
MIKE CREWE
Thelarge and highly diverse plant family, Caryophyllaceae, contains a great many species that we know as pinks, campions, chickweeds, catchflys, stitchworts and the like. Within this rather bewildering wealth of species sits a little group known as the mouse-ears, in the genus Cerastium.
The mouse-ears get their name from their noticeably hairy leaves, which are usually simple, rounded and do indeed look like little mouse’s ears on some species. This overall hairiness sets them apart from otherwise rather similar groups such as chickweeds and stitchworts and so is a good starting point for identification.
Mouse-ears have white flowers with (usually) five styles and four to five, free petals. The petals are partly divided or notched at the tip. There are 11 species currently mapped on the BSBI website and covered in Stace’s flora; three species are of higher altitude, largely confined to Scotland but also rare in Wales and Northern England; these are Alpine Mouse-ear (Cerastium alpinum), Arctic Mouse-ear (C. nigrescens) and Starwort Mouse-ear ( C. cerastioides). Dwarf
Little Mouse-ear (Cerastium semidecandrum). John Norton

Mouse-ear (C. pumilum) is a rather localised native of chalky grassland from the south coast northward to the Cotswolds and Northamptonshire; Grey Mouse-ear (C. brachypetalum) is a rare introduction, perhaps currently known from just two locations and Snow-in-summer (C. tomentosum) is a common garden escape, but rather different to the other mouse-ears in having densely white-hairy stems that creep to form large patches and unlikely to be confused with the native species covered here. This leaves us with a manageable five species likely to be encountered by beginners throughout much of Britain and Ireland (though some are rather local in Ireland) with Sticky, Little and Sea Mouse-ears being the three that can be most confusing. Spring is the time to look for these little gems, with some species starting to flower as early as March.
The five commoner species
Each of the five commoner species is described below, but it is worth first noting their habitat choice and growth style. We have two species that
BSBI NEWS 152 | January 2023 37
BEGINNER’S CORNER: Making sense of mouse-ears
have relatively narrow leaves and which are both perennials; as perennials, they consistently have leafy stems without flowers, which spread to form creeping mats. Also, as perennials, they are typically found in established grassland habitats rather than as plants of disturbed, open ground. The other species are all annuals and are typified by being small (sometimes very small!) and consisting of a basal rosette of leaves that eventually produces a cluster of flowering stems. These annual species are usually to be found on disturbed or open ground and are common either as weeds of gardens, allotments or arable farmland, or on gravel drives, walls, beaches and bare, sandy ground.

To distinguish mouse-ears, careful attention to the flowers (including both the petals and sepals) and their bracts is necessary, so it is important to understand these structures. The flowers are carried in small clusters and may be in tight heads or carried more openly on longer pedicels (stalks). At the base of the flower pedicels will be found the bracts, which resemble very small, stalkless leaves. A hand lens may be required to be sure of getting a proper look at the margins of these bracts and compare them with the descriptions given for each species below.



 Mike Crewe mikedcrewe@gmail.com
Mike Crewe mikedcrewe@gmail.com
38 BSBI NEWS 152 | January 2023
Common Mouse-ear (Cerastium fontanum). A perennial of grassy places, from lawns and grazed pastures to water meadows and also as a short-lived species on cultivated ground. Leaves have a rich, almost emerald green look to them and form low, spreading mats, from which the flowering stems arise. Flowering branches are usually rather open and the petals about as long as or only slightly longer than the sepals. Lower flower bracts are usually entirely green and leaf-like, while the upper ones have a narrow, whitish, papery margin. Photos: Debbie Allan (top left); Mike Crewe (top right and other images).
Field Mouse-ear (Cerastium arvense). A perennial of light sandy soils, occurring on heathland, sand dunes, arable field margins and also chalk grassland (where it typically grows on ant-hills). Rather uncommon or localised in lowland Britain and absent from the west and much of Ireland. It forms creeping mats studded with large flowers more than 1 cm across with rather broad and overlapping petals and is distinctive among the species covered here in having narrow and rather sharply pointed leaves. Photos: Mike Crewe.




Sticky Mouse-ear (Cerastium glomeratum). An abundant annual in human-influenced habitats, including urban and agricultural environments. Leaves relatively broad and rounded, often pale green; bracts entirely green and leaf-like. Plants have glandular hairs but despite the name are usually not noticeably sticky, perhaps because of the presence also of long, silky, non-glandular hairs. These project beyond the tips of the sepals. After flowering, the seed capsules are carried on stalks that are shorter than the sepals. Photos: Mike Crewe.


BSBI NEWS 152 | January 2023 39
BEGINNER’S CORNER: Making sense of mouse-ears
BEGINNER’S
Little Mouse-ear (Cerastium semidecandrum). Widespread and locally common in lowland habitats, often forming dense colonies of tiny plants on open ground, especially where rabbits graze. Flowers with rather ragged and uneven petals, always in fives and with a shallower notch than the similar Sea Mouse-ear. Petals are shorter than the sepals and there are five stamens. The flower bracts have broad papery margins that account for at least a third of the length of the bract. Photos: John Norton (left, top right); Mike Crewe (bottom right).




Sea Mouse-ear (Cerastium diffusum). Widespread and quite common in coastal habitats, but also spreading inland along the margins of salted roads and occasionally on inland heaths and other disturbed habitats. Flowers are unique in the group in usually having only four petals, these being about as long as the sepals, but most populations will also have some flowers with five petals (top right). The flower bracts only have narrow papery margins, most obvious only at the tip. Leaves, stems and bracts/sepals are strongly glandular.

Photos: Debbie Allan (left, bottom right); John Norton (top right).

40 BSBI NEWS 152 | January 2023
CORNER: Making sense of mouse-ears
ADVENTIVES & ALIENS
Adventives & Aliens News 28
Compiled by Matthew Berry Flat 2, Lascelles Mansions, 8–10 Lascelles Terrace, Eastbourne, BN21 4BJ m.berry15100@btinternet.com
Itis with considerable pleasure that I include a record for Sagittaria subulata (Narrow-leaved Arrowhead) at Shortheath Common (v.c. 12), after having previously reported its very probable extinction at the site in 2017 (see Adventives & Aliens News 11, preamble, BSBI News 135: 67).
Similarly, it was a considerable relief to see a couple of very recent records for Carduus pycnocephalus (Plymouth Thistle) at Plymouth Hoe (v.c. 3) in the BSBI Distribution Database (DDb), after what seemed to be a bit of a scare c. 2016 (see Adventives & Aliens News 10, preamble, BSBI News 134: 40).
The only other point I would make concerns Chenopodium berlandieri (Pitseed Goosefoot). There is some small suggestion that it might be on the increase (see v.c. 56). To make sure it is not evading detection as ‘odd’ C. album (Fat-hen) the careful microscopic examination of fruiting perianth and seed testa will be necessary.
V.c. 3 (S. Devon)
Nigella hispanica L. (Fennel-flower). Newton Abbot (SX847728), 16/6/2022, R. Smith, J. Day & P. Sansum (det. J. Day): in a reseeded roadside below Highweek. It differs from the much more familiar N. damascena (Love-in-a-mist) in having flowers that lack a leaf-like involucre and follicles fused for c. two-thirds of their length (vs fused to tip); and from N. gallica Jordan (Pale Fennel-flower) in its densely glandular follicles and larger flowers (4–5 cm vs 2–3.5 cm) that are bright blue (vs pale blue to whitish). The only ‘modern’ record I can find details for is one on a Guildford refuse tip (v.c. 17) from 1975, Leslie (1987). A bird-seed casual, it has also been a garden annual and can presumably be
a constituent of wild flower mixes. Clement et al. (2005): 9.
Aptenia cordifolia (Heart-leaf Iceplant). Dawlish (SX96587689), 19/8/2022, P. Pullen (conf. I. Bennallick & D. Cann): naturalised on sea cliffs. New to v.c. 3. A perennial, S. African succulent (Aizoaceae) of spreading habit that has ovate, papillose leaves and purplish-red flowers up to c. 2 cm across. A not frost hardy garden plant established in coastal sites

BSBI NEWS 152 | January 2023 41
ADVENTIVES & ALIENS: Adventives & Aliens News 28
Papaver atlanticum, Northam, North Devon (v.c. 4). Bob Kirby
in v.cc. 1a, 1b, 10 and 113. Clement et al. (2005): 39. Stace (2019): 531.
V.c. 4 (N. Devon)
Papaver atlanticum (Atlas Poppy). Northam (SS45112952), 10/5/2022, R.I. Kirby (comm. R.I. Kirby/conf. M. Berry): c. 30 plants (flore pleno) spread along c. 20 m of pavement. New to v.c. 4. An orange-flowered Moroccan perennial (Papaveraceae) that readily escapes from gardens and often persists. Earlier records of the rusty-red flowered Armenian native P. lateritium were errors for this. Clement et al. (2005): 18. Stace (2019): 93.
V.c. 5 (S. Somerset)
Euphorbia maculata (Spotted Spurge). Willowbrook (ST12), 26/6/2022, S.J. Leach (comm. S.J. Parker & S.J. Leach): several plants in paving in nursery area of garden centre. The first v.c. 5 record (and see below). There are already several records from nurseries and garden centres in v.c. 6. Adventives & Aliens News 27, v.c. 57.
Cardamine occulta Hornem. (Cryptic Bitter-cress).
Minehead (SS94), 22/4/2022, G. Lavender (comm. S.J. Parker & S.J. Leach): weed in garden centre; Yeovil (ST51), 23/4/2022, I.P. Green (comm. S.J. Parker & S.J. Leach): weed in the Brimsmore garden centre. These are the first and second v.c. 5 records respectively. An Asian annual (Brassicaceae) with some 75 British and Irish records now to its credit, still predominantly as a weed of garden centres, nurseries, planted tubs and gardens. See Adventives & Aliens News 18, v.c. 13.
Saponaria ocymoides (Rock Soapwort). Taunton (ST22), 4/5/2022, S.J. Leach (comm. S.J. Parker & S.J. Leach): several plants on grassy verge of riverside path, Coal Orchard. The first v.c. 5 record. A perennial, mat-forming, mountain plant of southern Europe (Caryophyllaceae), that has small, scabrid-ciliate, ovate-elliptic leaves (the upper narrower and more acute than the lower) and lax terminal inflorescences of deep rose-pink flowers with unnotched petals, c. 10 mm across. It is a garden plant being particularly a favourite for rockeries. Stace (2019): 504.
Veronica peregrina (American Speedwell).
Willowbrook (ST12), 26/6/2022, S.J. Leach (comm. S.J. Parker & S.J. Leach): hundreds of plants in the paths at Willowbrook nursery and garden centre. The first v.c. 5 record in c. 20 years. An erect, glabrous, N. American annual (Veronicaceae), and classic nursery weed, that has very pale blue almost white corollas followed by hairless, bivalvate capsules that are both much shorter than the calyx lobes. Clement et al. (2005): 251. Stace (2019): 622.
V.c. 9 (Dorset)
Eschscholzia caespitosa Benth. (Tufted Californian Poppy). Swanage (SZ03307807), 27/8/2022, D. Leadbetter: a few plants on bank in lane near Sunnydale. New to v.c. 9. In the remarkably similar E. californica (Californian Poppy) after the hoodlike (calyptrate) calyx is shed, a collar-like structure
remains immediately below the flowers; this collar is absent in E. caespitosa. It is also generally smaller in its parts. There are three other records in the DDb for v.cc. 17 (2018), 39 (2007) and 67 (2019).
Trachelium caeruleum (Throatwort). Swanage (SZ02647973), 31/8/2022, D. Leadbetter: one on pavement in D’Urberville Drive. A glabrous perennial (Campanulaceae) native to southern Europe. The numerous lilac-blue flowers are densely clustered in branched, flat-topped, terminal inflorescences, each flower having a very slender corolla tube (6–8 mm

42 BSBI NEWS 152 | January 2023 ADVENTIVES & ALIENS: Adventives & Aliens News 28
Eschscholzia caespitosa, Swanage, Dorset (v.c. 9). David Leadbetter
long) and a limb of 5 short spreading lobes. The style greatly exceeds the corolla and there are 3 stigmas.
also accompanied by Amaranthus blitum (Guernsey Pigweed) and Solanum villosum (Red Nightshade). A glandular hairy, aromatic annual (Amaranthaceae) native to Australia, known as a wool alien in Britain and Ireland. Distinguished from D. carinata and D. cristata by its unkeeled tepals; from D. botrys by its shallowly toothed leaves (vs deeply, narrowly lobed); from D. ambrosoides by the presence of hairs (glandtipped or not) on the tepals (vs glabrous); and from D. multifida by the tepals lacking net-veining and being fused for less than half their length. For Graham Easy’s drawing of a Cambridgeshire plant see p. 8 of BSBI News 20. Clement et al. (2005): 48. Stace (2019): 521.
The leaves are elliptic-ovate with toothed margins, the upper ones narrower and having shorter petioles. A garden escape found growing on walls and the like. Clement et al. (2005): 266. Stace (2019): 711.

V.c. 10 (Isle of Wight)
Dysphania pumilio (Clammy Goosefoot). Ventnor Botanic Garden (SZ5476), 20/8/2022, C. Pope (det. E.J. Clement): in excess of 1000 plants growing through gravel along paths, with very luxuriant Portulaca oleracea (Common Purslane). First observed here in 2021 by Paul Stanley, when it was


BSBI NEWS 152 | January 2023 43 ADVENTIVES & ALIENS: Adventives & Aliens News 28
Dysphania pumilio (and Portulaca oleracea), Ventnor Botanic Garden, Isle of Wight (v.c. 10). Colin Pope
Trachelium caeruleum, Swanage, Dorset (v.c. 9). David Leadbetter
V.c. 12 (N. Hants)
Sedum hispanicum (Spanish Stonecrop). Aldershot (SU859509), 7/5/2022, P. D. Stanley (comm. A. Mundell): one clump at foot of bank near to the car wash at Morrisons supermarket. Since 2010 there have been numerous records for the usual habitats – on wall tops, at wall bases, from road sides, hard standing, pebble-set paving and a cliff edge – for v.cc. 5, 10, 13, 16, 17, 29, 33, 38, 44, 54, 64 and 95. See Adventives News 21, v.c. 13. Stace (2019): 150. Sagittaria subulata (Narrow-leaved Arrowhead). Shortheath Common (SU77533690), 12/7/2022, S. Povey (comm. A. Mundell): a splendid flowering patch at margin of large pond. An aquarists’ throwout (Alismataceae) from eastern N. America with submerged linear leaves and small white threepetalled flowers that open one at a time. As well as other Sagittaria species, A.L. Grenfell commented on its close resemblance to Luronium natans (Floating Water-plantain). Thought lost since c. 2008, it was rediscovered by Fred Rumsey in 2019. These plants are perhaps derived from surviving seed (or turions?) and for now the species is hanging on rather than flourishing. It is also known from v.c. 108 (record not in DDb). Stace (2019): 878.
V.c. 13 (W. Sussex)
Cuscuta campestris (Yellow Dodder). Billingshurst (TQ07962584), 5/9/2022, F. Abraham (comm. F. Abraham): in flower and bud sprawling over Polygonum (Knotweed), Helminthotheca echioides (Bristly Oxtongue) and Taraxacum (Dandelion) in an abandoned (soon to be built on), formerly arable field. A parasitic N. American native (Convolvulaceae) occurring as a ‘weed’ of cultivation and also one of a clutch of recurring Niger-seed aliens. See Adventives & Aliens News 3, v.c. 85. Clement et al. (2005): 227. Stace (2019): 603.

v.c. 14 (E. Sussex)
Reseda phyteuma (Corn Mignonette). Hampden Park (TQ61050210), 9/9/2022, M. Berry: a few scattered plants on disturbed ground by footpath, Hampden Retail Park. Associates included Persicaria amphibia (Amphibious Bistort/Terrestrial form), Atriplex patula (Common Orache), Lipandra polysperma (Many-seeded Goosefoot), Chenopodiastrum murale (Nettle-leaved Goosefoot/one plant) and Solanum nitidibaccatum (Green Nightshade). Coincidentally (or not) S. nitidibaccatum was an associate of R. phyteuma at the Peacehaven (v.c. 14) site written up by Alan Knapp in 2005 (BSBI News 99, pp. 50–51). A southern European annual (Resedaceae) with pendent capsules and greyish, rugose seeds (cf. smooth, shiny black seeds of Reseda lutea). In Sussex it has essentially been a semi-persistent weed of arable (wheat, maize). Clement et al. (2005): 119. Stace (2019): 410.
Solanum chenopodioides (Tall Nightshade). Eastbourne Old Town (TV59379983), 24/9/2022, M. Berry (conf. E.J. Clement): one plant among paviours, Fiennes Close. A South American perennial (Solanaceae) established in Guernsey (v.c. 113) since 1958 and in the Bermondsey area (v.c. 17) since 1989. It has also been known as a weed at RHS Gardens, Wisley (v.c. 17) since c. 1986. Elsewhere a rare if possibly increasing casual. The illustration on p. 215 of Clement et al. (2005) shows two helpful characters additional to those highlighted in Stace (2019) – strongly deflexed fruiting peduncles and styles that usually protrude well beyond the anther
44 BSBI NEWS 152 | January 2023 ADVENTIVES & ALIENS: Adventives & Aliens News 28
Sagittaria subulata, Shortheath Common, North Hampshire (v.c. 12). Steve Povey
tips (vs not or barely protruding in S. nigrum). Stace (2019): 611.
Artemisia campestris (Field Wormwood). Eastbourne (TV59479979), 21/6/2022, M. Berry (det. E.J. Clement): one plant (woody at base, branched low down and with many new shoots) in gutter of Mountney Road, Old Town. The subspecies has not been critically determined but the general habit and characters seem consistent with subsp. campestris. Enquiries made by Eric Clement suggest that A. campestris is just barely available as a foliage plant for gardens and this is perhaps the more likely explanation for its presence here, rather than an inadvertent memento of a stay in East Anglia. That it can tolerate life in gutters is known from observations at the Brandon Artemisia Reserve in Suffolk, see Marren (1999). The section of gutter containing the Artemisia was cleared just as capitula were beginning to show at the end of August/start of September, so no mature flowering material was ever collected (although it is possible that the root remains). Vegetative material is in herb. EJC. It is unusual for an Artemisia species in having leaves that are not at all aromatic. Stace (2019): 791.
V.c. 27 (E. Norfolk)
Crambe cordifolia (Greater Sea-kale). Cley (TG05324404), 18/7/2022, L. Parkerson: single plant close to exit of NWT visitor centre car park. The six-figure grid reference was obtained on a visit to the site by Bob Leaney and Jo Parmenter. First noted here in 2003 by Mike Crewe who also found a second plant on Sheringham Esplanade (TG15524351). The Cley plant has flowered and fruited but so far not produced any seedlings. These are the first and second Norfolk and v.c. 27 records respectively. A perennial garden plant (Brassicaceae) from the Caucasus, it has large, tough, coarsely lobed basal leaves with cordate bases and leafless panicles of small white flowers (c. 13 mm across). Stace (2019): 442.
Nemesia fruticans (Thunb.) Benth. (A Nemesia sp.). North Walsham (TG28513012), 20/7/2022, S. Pryce (det. M. Crewe): self-seeded into unmanaged paved area outside library, the source not or no

longer apparent. The first record for v.c. 27 and Norfolk. A S. African perennial (Scrophulariaceae) which probably often behaves as an annual when grown as a garden plant in Britain and Ireland. Distinguished from N. strumosa (Cape-jewels) by the presence of a distinct spur in place of the latter’s short, fat, pouch-like appendage. N. denticulata, which also has spurred corollas and the potential to be sub-shrubby, must come very close to it. I note that some Belgian botanists record escaped Nemesia taxa using ‘Nemesia-Hybrids’ as a cover-all name, on the assumption that very few in cultivation equate to pure species. More taxonomic work and detailed keys will be needed before we can tighten up naming of British and Irish escapes belonging to this difficult S. African genus.
V.c. 28 (W. Norfolk)
Rhododendron cuneatum W. W. Sm. (A Rhododendron sp.). Rougham (TF84651989), 3/5/2022, M. Crewe (comm. J. Parmenter & R. Leaney): well established in woodland but probably planted originally. First
BSBI NEWS 152 | January 2023 45 ADVENTIVES & ALIENS: Adventives & Aliens News 28
Nemesia fruticans, North Walsham, East Norfolk (v.c. 27). Suki Pryce
record for both v.c. 28 and Norfolk. A native of China (south Sichuan and north-west Yunnan). A much branched evergreen shrub (Ericaceae) to 2 m. The leaves (1–7 × 0.5–2.8 cm) are broadly elliptic to oblong-lanceolate, dull pale green above, uniformly brown below, cuneate to rounded at the bases and strongly mucronate at the tips. The inflorescences are of 1–6 flowers, the corollas funnel-shaped (2–3 cm long), deep purple to rose-lavender, their outer surfaces pubescent and there are 10 stamens.
V.c. 40 (Salop)
Lysichiton camtschatcensis (Asian Skunk-cabbage). Oswestry (SJ30782947), 1/6/2022, M. Duffell: one of a number of ornamental species introduced in Eaton Pond, where the first v.c. 40 record of the highly invasive Hydrocotyle ranunculoides (Floating Pennywort) was made in 2008 (Brian Laney). A rhizomatous east Asian native (Araceae), much rarer than its N. American relative L. americanus (American Skunk-cabbage), from which it differs in its unscented flowers and smaller white spathes. Stace (2019): 874.
V.c. 55 (Leics)
Sorghum halepense (Johnson-grass). Leicester (SK570060), 16/8/2022, R. Parry (conf. R. Gornall): several well-established clumps in ramp to unused garage, Newstead Avenue. The first v.c. 55 record. A rhizomatous perennial grass known from wool, bird-seed, oil-seed and also sometimes planted. A native of north Africa and south-west Asia, it has proved to be invasive elsewhere in the world but not in this country to date, where it is typically of casual occurrence, persisting for a few years at most.
S. arundinaceum (Desv.) Stapf can approach it in leaf width but is an annual or short-lived perennial that does not form extensive patches. A native over much of Africa (absent from the north), it was a cotton casual in Oldham (v.c. 59) in 1976. Clement et al. (2005): 416. Stace (2019): 1111.
V.c. 56 (Notts)
Cotoneaster astrophoros (Starry Cotoneaster). Mansfield Golf Course (SK575610), 13/6/2020, M. Woods: bird-spread onto access track from planted shrubberies in nearby, disused, club house car park. First v.c. 56 record. An evergreen shrub (Rosaceae) from China, with tiny, obtuse, elliptic-obovate leaves (5–9 mm long), tomentose below and one-flowered inflorescences succeeded by orange-red berries. Stace (2019): 243.
Malope trifida (Mallow-wort). Nottingham (SK54773633), 5/9/2020, D.C. Wood: one plant in arable field margin; alien associates included Malva trimestris (Royal Mallow), Verbena bonariensis (Argentine Vervain) and Erigeron sumatrensis (Guernsey Fleabane). First v.c. 56 record. See Adventives & Aliens News 27, v.c. 3.
Chenopodium berlandieri ( Pitseed Goosefoot). Worksop (SK58577861), 17/8/2022, G.L.D. Coles: base of street lamp at edge of a car park, Newgate Street. New to v.c. 56. See Adventives & Aliens News 25, v.c. 57.
V.c. 57 (Derbys)
Vicia benghalensis (Purple Vetch). Smalley (SK407449), 5/2018, S. Jackson: growing on waste ground (where possibly introduced on the wheels of lorries). An annual (Fabaceae) native to the Mediterranean region which has occurred as a wool and grain casual and garden seed impurity in Britain and Ireland. Rather like V. villosa (Fodder Vetch) it has fewer flowers per raceme and reddish-purple corollas (vs bluish) with blackish tips. For a photo see Derbyshire Flora Group Newsletter No. 27, p. 7. Adventives & Aliens News 21, v.c. 10. Stace (2019): 169.
Salvia hispanica L. (Chia). Wingerworth (SK36296718), 9/2022, M. Lacey (comm. M. Lacey): one non-flowering plant (identity confirmed from photo), Stubbing Court. Mick Lacey links its occurrence here with a pond where local people feed the ducks and other birds. See preamble of Adventives & Aliens News 26. The first v.c. 57 record.
46 BSBI NEWS 152 | January 2023 ADVENTIVES & ALIENS: Adventives & Aliens News 28
V.c. 63 (SW Yorks)
Chaenorhinum origanifolium (Malling Toadflax). Huddersfield (SE1319), 23/9/2022, J. Lucas (det. M.P. Wilcox/comm. L. Hill & K. Woodward): one plant in gap between pavement and garden wall, Cowcliffe. The first v.c. 63 record. The recorder subsequently found another plant nearby. See Adventives & Aliens News 22, v.c. 55.

Oenothera speciosa Nutt. (Mexican Eveningprimrose). Pollington (SE612201), 19/5/2018, M.P. Wilcox: in Pollington Water Tower Quarry. A perennial garden plant (Onagraceae), a native of Mexico and south-central USA, with slightly concave pale pink (initially white) flowers up to 7 or 8 cm across. See Adventives & Aliens News 8, v.c. 14.
V.c. 83 (Midlothian)
Euphorbia stricta (Upright Spurge). Auchendinny (NT25446210), 17/7/2022, R.I. Milne (comm. I.
Strachan): c. 20 plants on untended slope in front of house where building work underway; Bush (NT24806400), 30/8/2022, R.I. Milne (comm. I. Strachan): one plant among weedy species on mound by road end. The first and second v.c. records respectively. See Adventives & Aliens News 23, v.cc. 67 and 68. Stace (2019):360.


BSBI NEWS 152 | January 2023 47 ADVENTIVES & ALIENS: Adventives & Aliens News 28
Salvia hispanica, Wingerworth, Derbyshire (v.c. 57). Mick Lacey
Euphorbia stricta specimen showing fruits, Auchendinny, Midlothian (v.c. 83). Sue Jury
Chaenorhinum origanifolium, Huddersfield (v.c. 63). Jill Lucas
Echinochloa crus-galli (Cockspur). Silverknowes (NT19807770), 21/11/2022, S. Jury (comm. I. Strachan): on promenade by seafront wall. There are seven other records for v.c. 83 in the DDb, those for Leith Docks going back to 1976. An annual grass from the tropics, now a widespread alien in Britain and Ireland. Ryves et al. (1996), Figure 25. Stace (2019):1105.
V.c. 106 (E. Ross)
Senecio inaequidens (Narrow-leaved Ragwort). Invergordon (NH709684), 25/7/2022, B.R. Ballinger (comm. I. Strachan): on waste ground by docks. New to v.c. 106.
V.c. 110 (Outer Hebrides)
Escallonia × langleyensis (Langley Escallonia). Great Bernera (NB17753447), 23/8/2022, P.A. Smith: present on roadside bank where not thought to be planted, Leathad Sgealaval. The first v.c. record. A more or less evergreen garden shrub (Escalloniaceae, formerly in Grossulariaceae) that is a hybrid between the Chilean E. rubra and the Chilean/Argentinian E. virgata. For differences between it and E. rubra (Escallonia), see Stace (2019). Another example found two days later on a roadside at Cidhe Chirceboist (NB186353) was of more uncertain status (P.A. Smith pers. comm.). Stace (2019): 822.
Levisticum officinale (Lovage). Great Bernera (NB18353565), 25/8/2022, P.A. Smith: one plant by seashore, Cidhe Chirceboist. The co-occurrence of Buddleja davidii (Butterfly-bush), an uncommon escape in v.c. 110, indicated the dumping of garden waste. The first v.c. record. See Adventives & Aliens News 27, v.c. 14.
References
Clement, E.J. & Foster, M.C. 1994. Alien Plants of the British Isles. Botanical Society of the British Isles, London.
Clement, E.J., Smith, D.P.J. & Thirlwell, I.R. 2005. Illustrations of Alien Plants of the British Isles. Botanical Society of the British Isles, London.
V.c. 96 (Easterness)
Cotula alpina (Upland Leptinella). Cawdor Castle (NH847498), 21/6/2022, I.P. Green (comm. A. Amphlett): patch on gravel and several patches in lawns. ‘First v.c. 96 records and first Scottish records away from north-west Highlands, where it is wellestablished in v.c. 105, with one site in v.c. 108’, in the words of Andy Amphlett, who communicated the record. See Adventives & Aliens News 18, v.c. 64.
Leslie, A.C. 1987. Flora of Surrey Supplement and Checklist. A.C. & P. Leslie, Guildford.
Marren, P. 1999. Britain’s Rare Flowers. T. & A.D. Poyser Ltd, London.
Poland, J. & Clement, E.J. 2020. The Vegetative Key to the British Flora (2nd edn). John Poland, Southampton.
Ryves, T.B., Clement, E.J. & Foster, M.C. 1996. Alien grasses of the British Isles. Botanical Society of the British Isles, London.
Stace, C.A. 2019. New Flora of the British Isles (4th edn). C & M Floristics, Middlewood Green, Suffolk.

48 BSBI NEWS 152 | January 2023 ADVENTIVES & ALIENS: Adventives & Aliens News 28
Echinochloa crus-galli, Silverknowes, Midlothian (v.c. 83). Sue Jury
Does Doronicum × longeflorens occur in Britain and Ireland?
ERIK CHRISTENSEN
In three decades of floristic survey campaigns in the district of Plön (Schleswig-Holstein, Germany), several different alien Doronicum taxa (Leopard’s-banes) have been found. In addition to Doronicum columnae, D. × excelsum, D. pardalianches and D. × willdenowii, a formerly undescribed, sterile taxon has been observed. This was identified as Doronicum columnae × D. pardalianches, a taxon new to science, recently named Doronicum × longeflorens E.Chr. (Willer et al., 2022).
How to recognise D. × longeflorens
D. × longeflorens has features of D. columnae and D. pardalianches, but resembles D. columnae much more than D. pardalianches. The main distinguishing characters are summarised in Table 1.
• Doronicum columnae, D. pardalianches and D. × longeflorens have basal leaves with distinctly heart-shaped bases.

• Doronicum × excelsum, D. plantagineum and D. × willdenowii have basal leaves with at best weakly heart-shaped bases; in these taxa the shape of the leaf base is otherwise decurrent, cuneate, rounded or truncate.

D. × longeflorens is the most common neophytic Doronicum taxon in the district of Plön and has also been found in other parts of Schleswig-Holstein, in North Rhine-Westphalia and Rhineland-Palatinate. In many cases it is fully naturalised.
The identification keys in Leslie (1991), Edmondson (2000) and Stace (2019) are, in my opinion, in principle appropriate for identification of Doronicum species. However, the full variation and the overlapping of the key characters of the different taxa are not always properly described. It is of significant interest whether Doronicum × longeflorens, which is obviously not yet included in the keys, also occurs as a naturalised neophytic taxon in Britain
BSBI NEWS 152 | January 2023 49
ADVENTIVES
Doronicum × longeflorens, whole plant and basal leaf. Erik Christensen
&
ALIENS: Does Doronicum × longeflorens occur in Britain and Ireland?
ADVENTIVES & ALIENS: Does Doronicum × longeflorens occur in Britain and Ireland?
and Ireland. I would be interested to hear from anyone who thinks they may have recorded it.
References
Edmondson, J.R. 2000. Doronicum Linnaeus. In: Cullen, J. et al. (eds.): The European Garden Flora, Vol. VI. pp. 637 638. Cambridge.
Leslie, A.C. 1981. A note on naturalized Doronicum in Britain.
BSBI News 27: 22 23.
Stace, C. 2019. New Flora of the British Isles (4th edn). C & M Floristics, Suffolk.
Willer, J., Christensen, E., Wahl, A., Gemeinholzer, B.& Zidorn, C. 2022. Phylogeny and chemophenetics of the newly described Doronicum × longeflorens and related Doronicum taxa (Senecioneae, Asteraceae). Biochem. Syst. Ecol. 101(2): 104400, DOI: 10.1016/j.bse.2022.104400
Erik Christensen
Masurenweg 22, D 24253 Probsteierhagen, Germany erik.christensen@gmx.de
Table 1. Key to morphological characters of Doronicum × longeflorens and its parental species. The leaves are only suitable for identification during the spring to summer rest period. The flowering period refers to Schleswig-Holstein (Germany).

Character D. columnae D. × longeflorens D. pardalianches
Upperside of the blades of the basal leaves
Sinus of the leaf lobes of the basal leaves
Rhizome appearance

Nearly glabrous, hairs about 0.1 mm long
Distinctly hairy, hairs 0.5–1 mm long
Distinctly hairy, hairs 0.5–1 mm long
Open Open Almost or completely closed
More or less cylindrical, incisions barely visible
Production of stolons Not producing underground stolons
Coverage of the swollen parts of the rhizome
Almost entirely covered by brown or dark leaf sheath remains
Consists of swollen regions, clearly separated from each other
Consists of swollen regions, clearly separated from each other
Sometimes producing short underground stolons Producing long underground stolons
Only narrow, ring-shaped remnants of the leaf sheaths
Only narrow, ring-shaped remnants of the leaf sheaths
Flowering period April to mid/end of May April to mid-June Mid-May to early July
Fertility/sterility
Fertile
Sterile
Fertile
50 BSBI NEWS 152 | January 2023
Doronicum × longeflorens, flowering plant (left) and rhizome (above) showing the remains of old leaf sheaths. Erik Christensen
Artemisia austroyunnanensis Y. Ling & Y.R. Ling (‘Giant Mugwort’) in v.c. 16 (West Kent)
 RODNEY BURTON
RODNEY BURTON
Ata meeting of the Kent Botanical Recording Group, led by Sue Buckingham and myself, on 4 August 2016, 13 members assembled in the private car park of a shooting club near the northern corner of Dartford Marshes (v.c. 16, W. Kent). Because of its remoteness this area had few recent records compared with the Crayford side of the tidal river Darent which has a much used right of way, but it was known to have several habitats with great potential. The car park was not one of these habitats, and I was eager to leave it, but one extraordinary plant commanded attention. Its great height and width were evident in comparison with Helminthotheca echioides (Bristly Oxtongue) and Tripleurospermum inodorum (Scentless Mayweed) in front of it ( see photo), but the detail of the capitula and foliage showed that it was closely related to Artemisia vulgaris (Mugwort). Just as the party was about to move off, I discovered a much smaller and evidently younger specimen perhaps 60 metres away, presumably a seedling, which indicated the branching pattern of the plant. It was single-stemmed for about 15 cm, then immediately forked two or three times into branches growing almost horizontally for a short distance before turning to vertical. Presumably further branching of the plant will result in its shape at anthesis.
I had already made the acquaintance of Filip Verloove before he published an account of Chinese Artemisia species naturalised in the Benelux countries, so it was to him that I sent photographs of the unknown plant as email attachments, and Sue sent a pressed specimen by post. He was probably far away collecting specimens for the Belgian national herbarium, and I can forgive him for his failure to connect the two items lurking in his inbox and intray. As a precaution, I went back to the car park on 27 October to collect more specimens, which I was
unable to do because all plant material there had been removed. Instead there was a tarmac surface covering the entire area of the car park.
Much later I began my search for a name for this plant. No European species came anywhere near fitting, and it was only when a kind friend told
BSBI NEWS 152 | January 2023 51
The main plant of Artemisia austroyunnanensis at Dartford Marshes (v.c. 16), 4 August 2016.
ADVENTIVES & ALIENS:
Y.
& Y.R. Ling in v.c. 16
Lliam Rooney
Artemisia austroyunnanensis
Ling
me about the online Flora of China that I soon found the answer (Ling et al., 2008). Enough of the characters needed to run the plant down to Sect. Artemisia could be found in the photographs. The section has 56 species in China, 28 of which are endemic, but fortunately at an early stage the key to species separates a single one on the character of its great height. Details are as follows:
Artemisia austroyunnanensis Y. Ling & Y.R. Ling, Bull. Bot. Res. Harbin 4(2): 20 (1984)
Subshrub 150–200 cm or more. [The leaves of the young plant, which may differ from those of the mature plant, are not described.] Synflorescence a broad panicle. Capitula many, nodding, involucre oblong or ovoid-oblong, 1.5–2.5(–3.5) mm. …
Grasslands, slopes, shrubland, forest margins, canyons, 800–2300 m. S & SW Yunnan (Bhutan, N. India, N. Myanmar, N. Thailand, N. Vietnam).
(I have omitted descriptions of floral features which cannot be confirmed from the photographs, and synonyms in the form of varieties of two different species, described by Pampanini.) If an English name is needed, I suggest ‘Giant Mugwort’.

How this plant could have travelled from Asia to Dartford Marshes is a mystery. The site is only about 800 metres from the tidal Thames, but it is hard to imagine how plant material could have been transferred accidentally from a ship. A less unlikely source is ground about 1500 metres to the south, where the pharmaceutical company Burroughs Wellcome was growing plants for experimental material during the Second World War, from which Digitalis lanata Ehrh. escaped, becoming naturalised until at least 1952 (Burton 1983: 123). Many Artemisia species are known sources of sesquiterpene lactones, which are widely used in lotions, perfumes, cosmetics, ointments and topical medications, and A. austroyunnanensis, which will grow rapidly to provide large amounts of source material, has since been tested for the availability of these compounds (Lan Lui et al., 2020).
Acknowledgments
Eric Clement gave me helpful advice on where to look for possible identifications of this plant. Lliam Rooney took many photographs at my behest.
References
Burton, R.M. 1983. Flora of the London Area. London Natural History Society: London.
Liu, L., Liu, D., Xiang, C., Dai, W., Li, B. & Zhang, M. 2020. Sesquiterpene lactones from Artemisia austroyunnanensis suppress ROS production and reduce cytokenes, iNOs and COX–2 levels via NF-KB pathway in vitro. Nat. Prod. Res. Jun. 34 (11): 1563–66.
Ling Y.-R., Humphries, C.J. & Gilbert, M.G. 2008. 151 Artemisia Linnaeus Sp. Pl. 2: 245, 1753. In Flora of China, Vol. 20–21: www.efloras.org
Rodney Burton
40 Pollyhaugh, Eynsford, Dartford, DA4 0HF rodneyburton@plus.com
52 BSBI NEWS 152 | January 2023
Flowers of Artemisia austroyunnanensis. Lliam Rooney
ADVENTIVES
& ALIENS: Artemisia austroyunnanensis Y. Ling & Y.R. Ling in v.c. 16
Cicerbita macrophylla subsp. macrophylla (Blue Sowthistle) in v.c. 64 (M.W. Yorkshire)

 HOWARD M. BECK
HOWARD M. BECK
The nineteenth of April 2019 was a day not unlike any other for a field botanist collecting records for Atlas 2020. The usual Pennine weather prevailed. The Covid pandemic had yet to fall on Britain like the Sword of Damocles, yet as the deadline for submitting records approached field activity was becoming ever more frenetic.
My day in the Yorkshire Dales was drawing to a satisfying close. I had garnered what I considered a respectable collection of observations for a single outing; however, during the drive home decided on a whim to dip into the pretty village of Clapham (SD7469), ancestral seat of the Farrer family and one-time home of Reginald Farrer (1880–1920), botanist and famed plant collector.
I have known the village over fifty years, grown familiar with its leafy milieu and its boisterous beck. I was aware that on its west and south-west sides the main car park is defined by a margin of unkempt ground – dominated by Urtica dioica (Common Nettle), Chamaenerion angustifolium (Rosebay Willowherb) and Rubus agg. (bramble) – that separated it from the footpath I have often taken to the neighbouring village of Austwick. On this occasion I came upon a patch of leaves I was unable to recognise. Photographs were taken and forwarded to the v.c. 64 Recorder, David Broughton, who informed me it was Cicerbita. I thought little more of it.
Three months later, on the overgrown east bank of the beck running through Clapham, David himself discovered a blue sow-thistle with a growth pattern that did not seem quite right. It was a more robust, statuesque plant up to two metres or more tall, had a dense fuzz of glandular hairs in the inflorescence and basal leaves with outrageously large terminal lobes. A little research and a visit to the online Manual of the Alien Plants of Belgium determined that these plants
BSBI NEWS 152 | January 2023 53
ADVENTIVES & ALIENS:
v.c. 64
Cicerbita macrophylla subsp. macrophylla (Blue Sow-thistle) in
Cicerbita macrophylla subsp. macrophylla (Blue Sow-thistle) at Clapham, Yorkshire (v.c. 64), 2019. Above: plants growing with nettles by the car park; below: a large basal leaf. Howard Beck
Cicerbita macrophylla subsp. macrophylla (Blue Sow-thistle) showing glandular hairs on flower stalks and phyllaries. Howard Beck
were Cicerbita macrophylla subsp. macrophylla. I knew nothing of this until I read David’s website blog. Afterwards I returned to the plants I had found beside the car park. By this time they were fully grown and blooming and immediately it was obvious these too were subsp. macrophylla, instantly
catapulting the Easter find into the fore as the first ever record for the entirety of Britain and Ireland. For further details of David Broughton’s record see Adventives & Aliens News 23 (BSBI News 147, April 2021), pp. 46–47.
These finds beg questions as to their origin. Reginald Farrer is known to have been an avid collector who returned with many exotic species for his private garden at Ingleborough Hall. Moreover, the Gunnera (Giant-rhubarb), bamboos and Petasites japonicus (Giant Butterbur) seen in the Ingleborough grounds are some of his introductions. A conversation with one of Reginald’s descendants, Philip Farrer, also of Clapham, revealed that the village car park is sited on land once occupied by the Ingleborough Hall greenhouses, bringing nearer the possibility that the Cicerbita was introduced by Reginald Farrer. Was he also responsible for the Cicerbita? We may never know.
Howard M. Beck
34 Varley Street, Colne, Lancashire, BB8 0RB
infinite_blue123@proton.me
Dispersal of Oxalis corniculata (Procumbent Yellowsorrel)
RODNEY BURTON
In the caption to the photograph of this species, the authors of the report from the first three years of Plant Alert (Dehnen-Schmutz et al., 2022) indicate that it has entered participants’ gardens accidentally in pots with other plants or with building materials or compost. I am confident that in my garden it has arrived, not by any of these means, but by seed. In 2019 the unmanageably steep lawn in the front garden of 44 Pollyhaugh uphill and upwind from me was replaced by cultivars of Erica × darleyensis (Darley
Dale Heath), and O. articulata was plentiful, no doubt accidentally introduced with the heathers. It was possibly present unnoticed here at no. 40 in 2020, but in 2021 there were several plants of it under tall and branchy Calendula officinalis (Pot Marigold) alongside the boundary fence with no. 42 and under nearby Bupleurum fruticosum (Shrubby Hare’s-ear). Although I had dug them out, many of the plants re-appeared in the same place in 2022, indicating that the species can regenerate from a fragment

54 BSBI NEWS 152 | January 2023
ADVENTIVES & ALIENS:
in v.c. 64
Cicerbita macrophylla subsp. macrophylla (Blue Sow-thistle)
of the slender vertical root left in the soil. More significantly, a single plant of O. articulata appeared in my back garden, which appears to be sheltered but can be reached by the wind rushing through the narrow space between nos. 40 and 42. Seeds of Epilobium spp. (willowherbs), Clematis vitalba (Traveller’s-joy), etc. also arrive by this route.
At some time longer ago, I had been struck by the way in which the capsules of O. articulata are held vertically on accrescent peduncles, rather like rockets waiting to be ignited in the course of a fireworks display. Now was the time to find out how this could be an adaptation to wind dispersal. Searching my own library found the answer in two places. Willis (1966) has this, relating to the genus as a whole:
‘The seed has a fleshy aril springing from the base. When ripe the cells of the inner layers are extremely turgid, and a small disturbance causes the aril to turn inside out, as one might turn a glove-finger from U to inverted U. This is done instantaneously and the seed is shot off.’
Demuth (1992) has a very much longer description of the process. What Willis calls an aril, he calls an exotesta. ‘The exotesta is divided into two layers; the layer nearer the centre of the seed is starchy and contiguous but not continuous from the testa and the hard outer layer. When the seeds are ripe and the capsule dehisces, the starch of the inner layer is somehow moistened and turns into sugar, causing an increase in the osmotic value and turgidity, and so there is a rapid increase in the tension of its tissues, which tears the hard outer layer in two at the back and pulls out the halves with great force’ (the inversion of the U in Willis’s description). I hope my translation does not betray my limited understanding of the catapulting process. I can see that a process using the seed itself might be more efficient, potentially dispersing numerous seeds in five different directions, corresponding to the five
loculi of the capsule, than some of the methods of catapulting seeds from a siliqua (Cardamine), legumes of many Fabaceae (especially Ulex where the process is audible) or capsule (most Viola). I cannot conceive how it could have evolved.

Acknowledgments
I must thank Eric Clement for his unstinting encouragement.
References
Dehnen-Schmutz, K., Kutlvašr, J. & Webb, A. 2022. Plant Alert – results from the first three years. BSBI News 151: 50–53.
Demuth, S. Oxalidaceae. 1992 Die Farn- und Blütenpflanzen Baden-Württebergs 4: 191–198. Stuttgart.
Willis, J.C. 1966. A dictionary of the flowering plants and ferns (7th edn, revised by H.K. Airy Shaw): p. 815. Cambridge University Press.
Rodney Burton
40 Pollyhaugh, Eynsford, Kent, DA4 0HF rmb@rodneyburton.plus.com
BSBI NEWS 152 | January 2023 55 ADVENTIVES & ALIENS:
Dispersal of Oxalis corniculata (Procumbent Yellow-sorrel)
Seedlings of Oxalis corniculata (Procumbent Yellowsorrel) in a discarded flower pot. John Norton
NOTICES
NOTICES
WELCOME TO OUR NEW STAFF
Awarm welcome to three new staff joining our team this winter.
James Harding-Morris is BSBI’s Countries Support Manager (full time). James previously worked for RSPB, including leading the Back from the Brink’s communications and engagement programme across 19 multi-species partnership programmes. James is a keen botanist and has recently been appointed as Vice-County Recorder for North Lincolnshire. He has a teaching qualification and a real passion for plants and engaging people in their stories.
Matt Harding is BSBI’s Scotland Officer (4 days per week). Matt was previously a self-employed environmental consultant and is a joint Vice-County Recorder for Stirlingshire. He also organises the local botany group and supports various other local volunteering and engagement work. Matt also has a teaching qualification.
Chantal Helm is BSBI’s Training Coordinator (2 days per week), working to coordinate Identiplant and FISC, funded by our new ways of working on these schemes. Chantal is also a Visiting Lecturer at the University of Hertfordshire and a freelance ecologist. She has previously worked in education for Cambridge University Botanic Garden and did a PhD on the autecology of a keystone tree species in the Kruger National Park.
James, Matt and Chantal all look forward to meeting you at future BSBI events and activities. In the meantime, do look out for interviews with them on the BSBI News & Views blog.
Julia Hanmer Chief Executive julia.hanmer@bsbi.org
2022 BSBI AGM
Once again the AGM was held online, allowing 88 members to attend, with one apology. All papers were available online and posted to members requesting them. Minutes are available on the BSBI website. The following resolutions were passed:
• The Minutes of the AGM held on 19 November 2021 were approved.
• The 31 March 2022 Annual Report and Accounts were adopted by the members.
• WMT LLP was re-appointed as Independent Examiner or Auditor, and Trustees were authorised to fix the remuneration of the Independent Examiner or Auditor.
• Prof. Peter Hollingsworth and Barry O’Kane were elected as Trustees.
• Dr Micheline Sheehy Skeffington was elected as President of the Society.
• Prof Ian Denholm was elected as an Honorary Member of the Society.
• The proposed new membership rates were approved.
I thank all members for attending and staff for making the event happen. Your feedback is most welcome.
Steve Gater Hon. General Secretary
steve.gater@bsbi.org
FROM THE MEMBERSHIP SECRETARY
Index to BSBI News
An index to BSBI News 131–140 will soon be available as a downloadable pdf on the publications section of the BSBI website – bsbi. org/publications/archive/bsbi-news-archive. Please contact me if you would like to obtain a printed copy of this or previous indices; only a limited numbers of copies will be available.
Address changes
When sending change of address details please remember to give your membership number or your old address, especially postcode and include any new phone number.
Gwynn Ellis Membership Secretary gwynn.ellis@bsbi.org
BRITISH & IRISH BOTANICAL CONFERENCE 2022
On 19 November, more than 200 botanists enjoyed the chance to meet up again in person at the Natural History Museum, London, for the British & Irish Botanical Conference (the event formerly known as the BSBI Annual Exhibition Meeting). The programme featured a 45-minute keynote talk from Chris Preston titled “How ‘natural’ are our alien species?” followed by eight 15-minute
56 BSBI NEWS 152 | January 2023
talks, celebrating the wild plants of Britain and Ireland and considering how plants help us and how we conserve them. The talks were recorded and the videos can be viewed on the BSBI YouTube channel.

There were 24 exhibits in total. Some provided the latest news about BSBI projects and activities, such as Identiplant, Field Identification Skills Certificates (FISCs) (both now run in-house), 2023’s programme of field and indoor meetings, and the forthcoming Plant Atlas. Recipients of BSBI grants exhibited posters outlining their research into subjects ranging from meadow monitoring in Derbyshire to plant diversity and soil carbon in woodlands; from plant communities of Strangford Lough islands to an examination of the Dryopteris affinis complex. Seven of the exhibitors also offered 1-minute flash talks about their posters, which proved very popular, as did ‘old favourites’ such as John Poland’s vegetative plant ID quiz and the behind-the-scenes tours of the NHM Herbarium.
The Conference webpage provides links to the videos, electronic versions of many of the exhibits, and photographs from the day: bsbi.org/british-irishbotanical-conference-2022

Many thanks to Sandy, Esther, Ilya, Chris and John at the Natural History Museum for hosting us, and to the Conference Organising Team, especially volunteers Andrew, Billy, George and Ryan from Events & Comms Committee.

Louise Marsh
BSBI Communications Officer louise.marsh@bsbi.org
NEW YEAR PLANT HUNT
By the time you read this, BSBI’s twelfth New Year Plant Hunt will be over, the results will be in and an analysis will have been carried out. You’ll be able to find out more in the April issue of BSBI News but if you can’t wait, please visit the New Year Plant Hunt webpage and follow the links for the Results map and the analysis. Many thanks to everyone who took part! Find out more on the webpage: bsbi.org/newyear-plant-hunt
Louise Marsh
MEETINGS
Enclosed with this issue of BSBI News you will find your copy of the 2023 Yearbook. This time it is a bumper edition with reports of meetings that took place in 2022 and the programme of meetings for 2023. Thanks are due to Gwynn Ellis for putting
BSBI NEWS 152 | January 2023 57 NOTICES
Some images from the British & Irish Botanical Conference 2022 held in November 2022 (see also p. 80). Louise Marsh
all the parts together. The reports are intended for the general reader, with the aim of persuading you to take part in a meeting, or perhaps to offer to host one yourself. Many of the regular fixtures have become social events as well as botanical explorations.
The big field meeting of the year is of course the Annual Spring/Summer Meeting, which this year is being held in Killarney. Outline details are in the Yearbook and by the time you read this it should be possible to register and pay online.
You will find the full programme of meetings on the field meetings and indoor events page in a form that allows you to search for particular types of meeting (Beginner, Recording, etc.). There are then links that allow you to email the organiser or to book for some residential meetings. We will keep the page updated throughout the season and may include meetings that missed the deadline for the Yearbook. Unfortunately, we cannot include all the local group meetings in this listing, so you will need to search through the county pages for meetings that you might want to attend.
To reduce our environmental impact we are no longer printing flyers for booking most residential and indoor meetings. If you don’t have access to the internet and do wish to come to a meeting you can book by phone by contacting Sarah Woods (details on the inside front cover).
Our series of virtual winter talks will again be held via Zoom on the first Wednesday of the month at 7.30 pm. The December talk described the latest research on the evolution and taxonomy of the Hyacinthaceae and there is a short account in the Yearbook. There are further talks on 1 February and 1 March, but if you missed earlier ones you can find recordings here: bsbi.org/winter-talks
I would like to thank all those who organise field meetings, which are much appreciated by members and supporters, both at national and local level.
Jonathan Shanklin
BSBI Hon. Field Meetings Secretary fieldmeetings@bsbi.org
COMMITTEE FOR ENGLAND
The 2022 English Botanical News was published on the BSBI website in May as a pdf for online reading. There is no printed version, but if you would like it in a form that you can print yourself as a booklet, please contact me. The 124 pages give you plenty to read and much to see as it is full of illustrations. View every issue here: bsbi.org/ publications/archive/english-botanical-news
The England Annual Meeting will take place as a Zoom meeting on 26 February. The meeting will include a short AGM followed by a series of talks with a theme of alien plants. All members and supporters are welcome to attend, but only those normally resident in England are allowed to vote should one be taken. You will be able to book from the England Annual Meeting page.
Following the main launch of Atlas 2020 on 8 March, there will be an England hybrid Zoom and in person meeting to celebrate the Atlas on 18 March. You will be able to find out more via the BSBI website and all members and supporters will be welcome to attend.
Looking further ahead, we are organising another Recorders’ Meeting at Preston Montford from 6–8 October. Anyone who records (whether as a ViceCounty Recorder, Referee or member) is welcome to come, though numbers will be capped at 60 participants. There will be a mix of talks, walks and workshops, making full use of the expertise of all the participants.
Jonathan Shanklin Chair, Committee for England
PANEL OF VCRS
Iam very grateful to Jo Parmenter who, while Pete Stroh is busy with the Atlas, put most of this together at the last minute with the help of Jim McIntosh, who sorted out the Scotland list. Contact details of Vice-county Recorders are given in the BSBI Yearbook, the new edition of which is enclosed with this issue of BSBI News. Please also refer to the Yearbook for up to date details of BSBI referees and specialists.
If you, or someone you know, is interested in taking up the role of VCR (or perhaps first trying it out as a trainee VCR), and would like to discuss what is involved, then please do get in touch with the relevant country officer (listed on the inside front cover) or for Wales please contact James HardingMorris, BSBI’s new Countries Support Manager.
John Norton (Editor)
England
We are delighted to welcome James Harding-Morris as VCR for North Lincolnshire (v.c. 54), who takes over from Paul Kirby, and John Martin and Mags Cousins in Shropshire (v.c. 40), who take over from Sarah Whild and Alex Lockton. Olga Krylova and Rupert Higgins have taken up posts as joint VCRs for West Gloucestershire (v.c. 34). We are also pleased to announce the appointment of a new joint recorder
58 BSBI NEWS 152 | January 2023 NOTICES
for North Devon (v.c. 4). Bob Kirby joins Jeremy Ison and Bob Hodgson, with the latter remaining the main point of contact. Paul Reade, VCR for Worcestershire (v.c. 37) has retired, leaving John Day in post. We were extremely sorry to hear of the death of Clive Lovatt, in West Gloucestershire (v.c. 34) and of Ian Hopkins, formally the VCR for Staffordshire. John Hawksford remains as the sole VCR for v.c. 39. Vacancies: There are currently vacancies in Surrey (v.c. 17), Essex (v.cc. 18 & 19), Buckinghamshire (v.c. 24) and Sark (v.c. 113). We continue to look for a joint recorder for Dorset (v.c. 9) to join Robin Walls.
Wales
We are pleased to announce the appointment of a new joint recorder in 2022: Heather Garrett in Merionethshire (v.c. 48). Jo Clark remains the main point of contact for the vice-county. Gillian Foulkes, joint VCR for Montgomeryshire (v.c. 47) has retired and we are grateful for her contribution since taking up the role in 2003. Her joint recorder, Kate Thorne, continues in post. There are no current vacancies, but see the note above if you are interested in helping out in Wales.
Scotland
We’d like to welcome two new VCRs to Scotland. Sue Jury in Midlothian (v.c. 83) succeeds Barbara Sumner, who, although officially retiring in late 2020, has been caretaking ever since. Sue has been very actively recording in the Lothians over the past five years and it is great to recognise that with this appointment. Flora Donald has been appointed VCR in Banffshire (v.c. 94), filling the vacancy that Andy Amphlett’s move to Easterness created in 2019. We are also pleased to announce the appointment of two new joint recorders in 2022: Joanna Walmisley in North Ebudes (v.c. 104) and Mary Dean in Easter Ross (v.c. 106). The main points of contact in these vice-counties will remain Stephen Bungard and Brian Ballinger, respectively. Malcolm Ogilvie, VCR for MidEbudes (v.c. 102) has retired and we are very grateful for his contribution to the BSBI since taking up the role in 2003. Although joint recorder Simon Smart takes over, Malcolm is happy to continue as unofficial local contact and help in whatever way he can. We were extremely sorry to hear of the deaths of Alastair Godfrey (v.c. 88) and Mark Tulley (v.c. 90), both of whom will be greatly missed.
Vacancies: Dave and Pat Batty in Kintyre (v.c. 101) and Paul Harvey in Shetland (v.c. 112) have all given notice that they would like to retire in 2023. Very kindly all three have agreed to ‘hold the fort’ until we can find successors. We continue to look for a joint recorder for Argyll (v.c. 98) and Midlothian (v.c .83).
Ireland
We are delighted to announce a number of new VCRs to Ireland. Julie Larkin, Andrew Malcom and Ann Trimble took up post together as joint VCRs for Co. Waterford (v.c. H6) and Tanya Slattery has taken over from Sylvia Reynolds in Co. Limerick (v.c. H8). Eamon Gaughan succeeds Don Cotton in Co. Sligo (v.c. H28). We were very sorry to hear of the sad death of Don in early 2022. He will be greatly missed. We are also pleased to announce the appointment of a new joint recorder in 2022: Ciarán Flynn has joined Kate Harrington in Co. Louth (v.c. H31) after Cliona Byrne stepped down from the role. Kate will remain the main point of contact.
Vacancies: There is a vacancy for a new VCR in Co. Cavan (v.c.H30) and Tanya Slattery is seeking a joint VCR for Co. Limerick (v.c.H28).
BSBI PLANT ATLAS 2020
The Atlas in print
We are delighted to announce that our longawaited third plant distribution atlas, Plant Atlas 2020: Mapping changes in the distribution of the British and Irish Flora will be published in two volumes in March 2023 by BSBI in partnership with Princeton University Press. Our members can benefit from an exclusive offer: from today until the end of March, you can claim a 50% discount when you order your copy, so you will pay £66 compared to the RRP of £132. This price is exclusive of postage & packaging, which will be £3.90 within the UK and £5.35 within the EU. A flyer inside this issue of BSBI News tells you more about the printed Atlas and explains how to claim your discount when ordering online – this information is also available on the password-protected members’ area of the BSBI website. For those members without internet access, please telephone Sarah Woods (contact details on the inside front cover of this issue) to find out how to order your copy.
Launching the Atlas project
Plant Atlas 2020 will be launched on 8 March 2023, with an online launch aimed at national media contacts and policy-makers. This will be followed by a series of face-to-face launches across Britain and Ireland: 9 March at the Royal Botanic Garden, Edinburgh and the National Botanic Gardens, Glasnevin, Dublin; 10 March at the National Botanic Gardens in Wales; 15 March at the National Museum of Northern Ireland; and 18 March at two locations in England: Cambridge and University of Newcastle.
BSBI NEWS 152 | January 2023 59 NOTICES
Keep an eye on our Atlas 2020 webpage for more details throughout February: bsbi.org/atlas-2020
The Atlas online
The online Plant Atlas also goes live on 8 March. This is a website, developed in partnership with UK Centre for Ecology & Hydrology, which features all the Atlas species data displayed in interactive maps, accompanied by text and graphics covering the phenology, altitudinal range and time-series trends for 2,863 native and alien taxa. Our Atlas 2020 webpage features sample pages from the print and online Atlases to give you an idea of what they will look like and how they will complement each other: bsbi.org/atlas-2020
Thank you!
There would be no third Atlas without the hard work of thousands of you who went out recording over the past 20 years and submitted your data. Thank you, we hope that you will enjoy seeing the outputs from all your hard work. Special thanks go to our County Recorders and expert Referees who collated and validated millions of records, and to BSBI staff members Kevin Walker, Head of Science, Pete Stroh, Scientific and England Officer and Tom Humphrey, Database Officer, who worked with Oli Pescott and Rich Burkmar at the UK Centre for Ecology & Hydrology to produce this authoritative survey of British and Irish wild plants. Thanks also to those who helped with editing captions and proof-reading.
Julia Hanmer and Louise Marsh
BSBI PHOTOGRAPHIC COMPETITION
Winners have been announced in the 2022 BSBI Photographic Competition and the 2023 Competition is already open for entries. The 2022 Competition attracted 194 entries in the categories ‘Plants in the Four Seasons’ from 77 keen botanical photographers. Many, but not all, were BSBI members: the Competition is open to everyone, whether or not they are members, and entry is completely free.
Winners were selected by a popular vote at the Scottish Botanists’ Conference, where attendees participants were able to view all entries as part of a mounted display comprising eleven A1 sheets which were then sent down to London to be viewed by attendees at the British & Irish Botanical Conference. The winners for 2022 were Alwyn Craven, for her photo of Hogweed, and Gillian Elsom who was lucky enough to be chosen as the winner of the other three categories with photos of Wood-sorrel, Fly Orchid and Autumn Lady’s-tresses. Congratulations
to both of them – we hope they enjoy their prizes of book tokens kindly donated by Summerfield Books.
For 2023, the categories are ‘Plants and People’ and ‘Plants in the Landscape’. To enter, please visit the Photographic Competition webpage bsbi.org/ bsbi-photographic-competition where you can read the rules and submit a photo. You will also be able to follow a link to the BSBI flickr page where you will find all the entries submitted over the last seven years. See p. 80 for a selection of this year’s entries.
We would like to extend our thanks to Natalie Harmsworth for her excellent work coordinating and promoting the Photographic Competition over the past seven years (thanks also to Claudia FergusonSmyth who helped me develop the competition eight years ago and who organised in that year).
Natalie is now ready to hand that role over to a new coordinator so if you are interested in taking up the reins, please email us at commsteam@bsbi.org to express an interest.
Jim McIntosh Outgoing BSBI Scotland Officer
IDENTIPLANT – CALL FOR TUTORS
BSBI has recently taken on the administration of the Identiplant online training course, for beginners who want to get started in serious botany. The course runs from February to September annually.
To continue the success of the course and reach more students, we are looking for new individuals to become Identiplant Tutors.
Tutors are experienced botanists familiar with their geographical area, who mark student answer sheets and provide feedback for improvement. You should be dedicated to the training of new botanists and happy to share your enthusiasm and expertise, as well as holding a FISC level 4 or equivalent, with good local field experience and able to communicate well in writing. The course is conducted entirely by email, with full materials and support provided by the Identiplant Management Team. Tutors are paid £150 for each student who completes at least one unit of the course.
To express an interest in becoming a tutor, please fill out the form available at identiplant.bsbi.org/ contact-us/becoming-a-tutor or email identiplant@ bsbi.org
Sarah Woods BSBI Fundraising Manager sarah.woods@bsbi.org
60 BSBI NEWS 152 | January 2023
NOTICES
LATEST WEBPAGES AND NEWS FROM BSBI
In this new regular section we are listing some of the pages on the BSBI website that you may be interested in having a look at.
• We’ve set up a ‘Jobs In Botany’ page to share details of jobs we hear about across the sector which might be of interest to botanists: bsbi.org/ jobs-in-botany
• Our Volunteering page has been updated recently, with new volunteering opportunities, some of which are exclusive to BSBI members: bsbi.org/volunteering-opportunities
• We’ve split our Training Courses page bsbi.org/ training-courses into two pages, internal and external, to reflect the increasing number of training courses and opportunities provided by BSBI – our webinars, online fern ID course, the playlist of short plant ID videos we’ve curated on our YouTube channel and the field meetings we offer with a training theme. To these we have just added Identiplant (see above) identiplant.bsbi. org and Field Identification Skills Certificates (see the article on page 34), both of which are now run by BSBI.
• We are also building links with botanical societies in other countries and to this end have updated our ‘International’ page and provided a link to the new Federation of European Botanical Societies, with whom we are starting to build strong links: bsbi.org/international
• We’ve also created new county pages – there is now a county page for every vice-county in England, Scotland and Wales, and for almost half the Irish vice-counties. For newcomers to botany and/or to BSBI, our Local Botany page, with links to all the county pages, and our Get Involved page with helpful hints about books, hand lenses, resources and support, are particularly useful. The best way to keep on top of all these webpages is probably via our A–Z Site Listing: bsbi.org/sitemap
To keep up with all the latest news from BSBI, please visit our News page and check out our midmonthly eNewsletter; you can also opt in to receive our Recorders’ eNewsletter for active recorders, or view current and back-issues.
Louise Marsh BSBI Communications Officer louise.marsh@bsbi.org
BRITISH & IRISH BOTANY 4:3
The third issue of the 2022 volume of British & Irish Botany, BSBI’s open access, online scientific journal, was published in September. You can view or download the papers free of charge, as well as previous issues and guidelines for submissions, from the B&IB website: britishandirishbotany.org/index. php/bib. You can also phone us on 07725 862 957 to discuss a proposal.
Please note that we are still looking to expand the Editorial Board and to find a replacement for the volunteer role of Editor-in-Chief, so please email us if you feel you have the skills and time, and are interested in joining the British & Irish Botany team.
Ian Denholm & Louise Marsh bib@bsbi.org
British & Irish Botany Vol. 4 No.3 (2022)
A review of the vascular plant flora of the Cairngorms Connect project area, Scotland, and some possible implications of forest expansion to the natural tree line – Andy Amphlett
Remarkable botanical records from Corrour in Westerness (v.c. 97), including Baldellia repens (Alismataceae) and Illecebrum verticillatum (Caryophyllaceae), new to Scotland – Sarah Watts, Ian Strachan, Richard Marriott
The history, status and conservation management of Cottonweed Achillea maritima (Otanthus maritimus) at Lady's Island Lake, Co. Wexford, Ireland – Tony Murray, Mike Wyse Jackson
Conservation of Britain’s biodiversity: Hieracium breconense (Asteraceae), Brecon Hawkweed – Tim Rich
Change in species distributions at tetrad scale – a supplement to Change in the British Flora 1987–2004 – Michael E. Braithwaite
The Deschampsia cespitosa (Poaceae) complex in Great Britain and Ireland – Hugh McAllister, Andy Amphlett
Cochlearia officinalis sensu lato (Brassicaceae) around northern Irish Sea coasts – Eric F. Greenwood, Hugh A. McAllister
A new spontaneous hybrid in Gunnera subgenus Panke (Gunneraceae) widespread in the British Isles, with notes on the typification of G. manicata – Julian M.H. Shaw, Dawn Edwards, John David Changing environment and orchid distributions close to their northern and southern limits in Britain (Correction) – David Trudgill
BSBI NEWS 152 | January 2023 61
NOTICES
COUNTRY ROUNDUPS
Compiled by Pete Stroh peter.stroh@bsbi.org
ENGLAND
Having been invited to write the report for this issue of BSBI News, we are unashamedly starting with some fern news. Firstly, it is with great excitement that we report the discovery by Louis Parkerson of a new sporophyte of Trichomanes speciosum (Killarney Fern) in West Cornwall (v.c. 1). Although global warming has seen the production of new plants from the gametophyte generation of this fern across its British range, this is one of only two extant populations of sporophytes in southern England (outside gardens). Another fern which is rare and declining in southern England is Gymnocarpium dryopteris (Oak Fern): this was found by Mark Gurney in 2016 new to Dorset (v.c. 9) and has recently been confirmed by FJR to be still present in plantation woodland near Arne. Those who attended the Irish Autumn Meeting or the British & Irish
Botanical Conference will have heard Alison Evans explain her PhD project on the Dryopteris affinis complex (see: bsbi.org/ irish-autumn-meeting-agm and bsbi.org/british-irish-botanicalconference-2022). Alison visited North Somerset (v.c. 6) with Roger Golding for two days of targeted site visits with us, during which they found Dryopteris kerryensis (Irish Male-fern) and D. pseudodisjuncta, both new to southern England. Subsequently,

Lionel Pike in South Devon (v.c. 3) has had a specimen found on Dartmoor confirmed by Roger as D. pseudodisjuncta and has subsequently found a second plant. This species was described by Ken Trewren (2014) as the scarcest species of fern in Britain: there are now three more known plants. Lionel has been studying this critical group of ferns in depth and has also found D. lacunosa (Pitted Male-fern) new to Devon and a new site for

62 BSBI NEWS 152 | January 2023
COUNTRY ROUNDUPS: England
Gymnocarpium dryopteris (Oak Fern), Arne, Dorset. Fred Rumsey
Alison Evans beside Dryopteris kerryensis (Irish Male-fern), Stockhill, Somerset. Helena Crouch
D. paleaceolobata. Meanwhile in South Somerset (v.c. 5) Graham Lavender has found D. × critica (D. filix-mas × borreri) at two sites on Exmoor, new to the county. For Leicestershire (v.c. 55), Geoffrey Hall reported a significant find of Samolus valerandi (Brookweed) on the bank of the Grand Union Canal. Jean Emeny discovered two plants where the Canal & River Trust had recently removed a tree, opening up the bank. This species has a strangely patchy distribution in England, largely coastal but with extensive concentrations of sites on the Somerset Levels and the Fens, yet absent from vast tracts of the country. The newly found site in Leicestershire, about 1 km downstream from a 1973 record, is only the third site for the county since 2000. Liz Askins wrote with news that another wetland species, Stellaria aquatica (Water Chickweed), which is surprisingly absent from parts of England, was found new to the Isles of Scilly in 2022 by Julian Branscombe and Pete Stroh. We were astonished to read that Liz herself had made the first record for ten years of Pale Persicaria (Persicaria lapathifolia) on the Isles of Scilly. It is always so interesting that one person’s common weed is another botanist’s rarity! Also on Scilly, a Persicaria species which will be less familiar to readers, found by Pete Stroh during survey work, has been identified as Persicaria odorata (Vietnamese Coriander), apparently new to Britain & Ireland. The large patch of plants, with impressively marked leaves was identified from Pete’s photo by Ian Benallick, and confirmed by John Akeroyd. It is grown as a culinary herb so worth looking out for elsewhere. An alien which has spread dramatically since the last Atlas, Euphorbia oblongata

(Balkan Spurge), has now reached Newcastle-upon-Tyne, where it was found new to South Northumberland (v.c. 67) by James Common, the new joint VCR for North Northumberland, whilst out urban botanising. Like Euphorbia oblongata, the dainty garden plant Chaenorhinum origanifolium (Malling Toadflax) had so few records before 2000 that it was only included on the CD which accompanied the last Atlas: it is now scattered across England and was found by Jill Lucas in a street in Huddersfield, new to Southwest Yorkshire (v.c. 63) (see Aliens & Adventives News, p. 47). By contrast, Anisantha tectorum (Drooping Brome) appears to be a declining neophyte. This was found by Steve Woodward in 2020 in a churchyard in Groby, Leicestershire (v.c. 55), the identification confirmed by Ken Adams at the recent BSBI Recorders’ Meeting: it is the only recent record for the Midlands.
Far from habitation, Vaccinium uliginosum (Bog Whortleberry) was refound at a site in South Northumberland (v.c. 67) where it had last been recorded in 1979. However, John Richards reports that pride of place for his county’s recent records should
go to David Feige’s discovery of Bistorta vivipara (Alpine Bistort) on gravels beside the South Tyne. This montane species was lost from its prime location in the county more than a decade ago, making this a very significant find. Another species refound in v.c. 67 was Epipactis phyllanthes (Greenflowered Helleborine), two spikes of which were recorded by Mark Welfare at the most northerly site in England, where it was last seen over twenty years ago. Meanwhile in Somerset, at its most southerly site in England, Viola lutea (Mountain Pansy) was refound by Ian Green at a location on Exmoor (v.c. 5) where it was last recorded (also by Ian) in 1999.
Refinding a species believed to be lost from a site is perhaps the most thrilling kind of record to make. In Surrey (v.c. 17) Ann Sankey reports the amazing discovery by Mick Rock of a vast population of Scilla autumnalis (Autumn Squill) at West Molesey. Mick found about 1000 plants flowering in an area of housing built on a former golf course, south of the known population at Hurst Park Racecourse. Brewer (1863) noted that H.C. Watson found this species in the field on the ‘contrary side of the road’ from Hurst Park: Ann wonders whether S. autumnalis has survived unrecorded here since c. 1850? This is now the largest known population in Surrey (see photos, next page). In Yorkshire, Kay McDowell reported the exciting discovery by Guy Wallbanks of Mentha pulegium (Pennyroyal) on the University of York campus. This is only the third site in South-east Yorkshire (v.c. 61) and the first record for the vice-county since 1937. It is the first record for York since 1863, when it was recorded at Haxby (v.c. 62). As a native, this species is Critically Endangered in England,
BSBI NEWS 152 | January 2023 63 COUNTRY ROUNDUPS: England
Stellaria aquatica (Water Chickweed), Isles of Scilly. Liz Askins
however it was reported in the last Atlas to be spreading as an alien introduced with grass seed (Preston et al., 2002); the status is thus often difficult to assess. When any attempt is made to distinguish between native and alien records, assumptions are made based on past known distributions and habitat preferences, but some plant species are opportunists and can adapt to new habitats. In South Northumberland (v.c. 67) John Richards reports that Polygonum rurivagum (Cornfield Knot-grass) has graced several upland salted road-verges in recent years, but in 2022 it appeared in some quantity along the North Shields esplanade, another saline site.

It is always deeply satisfying to announce the discovery of a species believed to be lost from a vice-county. In East Norfolk (v.c. 27) Bob Ellis reports that Orchis anthropophora (Man Orchid) turned up at the western edge of Norwich, the first record for v.c. 27 since 1950, a significant record for
this Endangered species. In South Somerset (v.c. 5), Simon Leach and a few Somerset Rare Plants Group friends found Oxybasis glauca (Oak-leaved Goosefoot) in a street in Taunton, new to the vice-county. This distinctive and attractive Goosefoot is Vulnerable on both the GB and England Red Lists. Another new vice-county record was reported by John Martin for Shropshire (v.c. 40), where Brett Westwood and John Bingham found Centaurium pulchellum (Lesser Centaury) at the edge of a track in Wyre Forest. Further good news for species of Gentianaceae was received from the North. In addition to news of Trifolium vesiculosum (Arrowleaf Clover), an alien new to Britain & Ireland (it is hoped an account will be included in a future issue of BSBI News), Dave Barlow reported the discovery of a few more sites for Gentiana pneumonanthe (Marsh Gentian) close to the exciting refind of this species mentioned in BSBI News 148. Meanwhile in South Northumberland (v.c. 67)
Gentianella campestris (Field Gentian) had a good year at its single remaining locality, with more than 100 plants flowering in four microsites.

Back in Somerset, in a disused quarry threatened with development, FJR found Euphrasia pseudokerneri f. elongata (Chalk Eyebright) in a small surviving fragment of fen, one of the few North Somerset
Euphrasia pseudokerneri f. elongata (Chalk Eyebright), Somerset. Fred Rumsey

64 BSBI NEWS 152 | January 2023
COUNTRY ROUNDUPS: England
Scilla autumnalis (Autumn Squill) colony, West Molesey, Surrey and Bombus vestalis (Vestal Cuckoo Bumblebee) visiting a flower. Mick Rock
(v.c. 6) sites for Epipactis palustris (Marsh Helleborine). This was only the second record of E. pseudokerneri in Somerset since 1830, and the first for the rarelyrecorded fen ecotype recognised by Peter Yeo (but not treated as distinct in the BSBI Handbook). Nearby, at Charlton Mackrell, HJC and FJR recorded many plants of Valerianella eriocarpa (Hairyfruited Cornsalad) in grassland
beside the railway, the first record for v.c. 6 since 2000. Growing on adjacent waste ground, were two plants of Silene viscaria (Sticky Catchfly), new to Somerset. As a native this is a rare and declining species but at Charlton Mackrell it is clearly an alien. Later in the year, John Poingdestre coincidentally found a single plant of Nepeta cataria (Cat-mint) on the same patch of waste ground. There have been no post-2000 records for this arable plant in Somerset. This record could be regarded as a casual garden escape like the Silene viscaria, yet Charlton Mackrell lies within the MidSomerset Important Arable Plant Area designated by Plantlife and Nepeta cataria was recorded at the same site in 1991, so clearly this is not a casual occurrence but a refind for Somerset of a Vulnerable archaeophyte, previously considered lost in the county.


We are hugely grateful to all those VCRs who sent us records for inclusion in this report. There have been some excellent discoveries in England this autumn and it seems that there is always something exciting to find, or indeed refind.
References
Brewer, J.A. 1863. Flora of Surrey
John Van Voorst, London. Trewren, K. 2014. Some taxa within the Dryopteris affinis complex: a field guide. British Pteridological Society, London. Preston, C.D., Pearman, D.A., & Dines, T.D. 2002. New Atlas of the British and Irish Flora. Oxford University Press, Oxford.
Helena J. Crouch
VCR for North Somerset (v.c. 6)
WALES
This round-up of Welsh plant news will be briefer than usual as a few recorders have not yet sent me their highlights for the late summer of 2022. I am grateful to those who have.
Kate Thorne and Gillian Foulkes noted that there have been some nice finds/refinds in Montgomeryshire (v.c. 47) since late summer mostly from the Montgomeryshire Canal and Severn floodplain. These wetland visits have been partly in preparation for our wetland themed BSBI AGM in 2023. Findings have included floating turions of Potamogeton compressus (Grass-wrack Pondweed) in the Montgomeryshire Canal in late September; this is a species that has become scarce (post 2000 assessment) in the canal whilst the all-important Luronium natans (Floating Water-plantain) appears to be locally frequent. Limosella aquatica (Mudwort) was refound along the Severn floodplain at three sites between Dolydd Hafren and Leighton this autumn.
There were also several good records for arable weeds mostly from floodplains but also from roadsides, with Rorippa islandica (Northern Yellow-cress, confirmed by Tim Rich) raised from county rare status (2 sites) to scarce (4 sites) and Hirschfeldia incana (Hoary Mustard) a new county record (confirmed by Mark Duffell).
The highlight of the year was a group visit to Trannon Moor to see Carex pauciflora (Fewflowered Sedge), first found here in 2000 by Ben Averis and refound in 2021. The stand proved much larger than expected because the time was right to see it. Chris Forster-Brown came to this visit as he has surveyed and monitored
BSBI NEWS 152 | January 2023 65
Valerianella eriocarpa (Hairyfruited Cornsalad), Charlton Mackrell, Somerset. Helena Crouch
Silene viscaria (Sticky Catchfly), Charlton Mackrell, Somerset. Helena Crouch
COUNTRY ROUNDUPS: England / Wales
Fred Rumsey Committee for England
this whole area for many years (re wind turbines), and had found another plant of interest here in 2003 – Sparganium natans (Least Bur-reed). Chris led us to ‘his’ stand of Sparganium, which is located 2 km due north of the Carex pauciflora area, passing through stands of Carex limosa (Bog sedge) and Carex magellanica (Tall Bog-sedge). Subsequently, a small quantity of Potamogeton alpinus (Red Pondweed) – confirmed by Chris Preston – was also found on the edge of this moorland, growing in a small pond (old man-made reservoir). This pondweed is listed as CR on the Welsh Red List and this is only the second county record, the other being from the Canal in 1986.
Sadly, the fond memories we have of our visit to Trannon Moor with Chris Forster-Brown have gained an unexpected poignancy from the recent news of his sudden death. This first sighting for all of us, of ‘his’ Sparganium is now his legacy to our small Montgomeryshire Flora Group. We also hope that this Sparganium area will become included into the SSSI which lies nearby and that the adjacent ravine (Nant Ysgolion, another of Chris’ favourite places) will also be included.
Chris also sent in several other good records from sites he had surveyed for work, whilst always managing to get into adjacent ravines and always including a bryophyte or two! His new tetrad records of particular interest were Vicia orobus (Wood Bitter-vetch) in SN9279 with Gymnocarpium dryopteris (Oak fern) and Phegopteris connectilis (Beech Fern) in SN8993.
In Cardiganshire (v.c. 46), Steve Chambers wrote that the Banc y Mwldan SSSI, c. 2 km northeast of Cardigan town, holds the
largest area of calcareous fen in the county and with it several rare plants. Howard Williams and Ruth Harding, while visiting the fen to update records for the Rare Plant Register (RPR), spotted a spike of a puzzling orchid. Initial investigations suggest it is a strong candidate for the intergeneric hybrid × Dactylodenia ettlingeriana (Dactylorhiza × Gymnadenia), which would be the first v.c. record. It has been duly flagged for a priority follow-up visit in 2023. Also on ‘mission RPR update’, Tim Rayner and Steve Chambers found new sites for Elatine hexandra (Six-stamened Waterwort), at Pond Rhosrydd and in a small hilltop pool near one of its strongholds below Banc Llwynwnwch.
Yusef Samari has also been active in the Cardigan area, finding new sites for vice-county scarcities, including Erigeron acris (Blue Fleabane), while in the hills Dylan Davies has been revisiting all the known historic sites for Rubus saxatilis (Stone Bramble), one of which appears to

have been destroyed by ‘forestry operations’.
Richard Pryce wrote that in Carmarthenshire (v.c. 44) recording visits had been made to most parts of the county during the year. However, it was very sobering to find that several uncommon species targeted for monitoring were not refound, despite observers being able to return to their precise locations. A number of factors may have contributed to their demise, probably most significantly, atmospheric pollution, habitat management changes and habitat loss due to neglect or agricultural improvement. Our findings are symptomatic of the ‘Nature Emergency’ declared by the Welsh Government, that our wildlife is facing and which appears to be becoming more severe at an ever-increasing rate.
In Glamorgan (v.c. 41), new hectad records have been made for four v.c. rarities – Sison segetum (Corn Parsley) near the Vale coast and Hymenophyllum wilsonii (Wilson’s Filmy-fern) on shaded boulders below a cliff
66 BSBI NEWS 152 | January 2023
Sison segetum (Corn Parsley), Glamorgan. David Barden
COUNTRY ROUNDUPS: Wales
in the uplands, Carex punctata (Dotted Sedge) on an artificial dune slack created on top of Margam landfill site and Vulpia ciliata (Bearded Fescue) on furnace slag in Swansea. Also in the last two years there have been records for Mentha pulegium from two colliery spoil sites, which like two other records made in the last 30 years appear to be the introduced var. erecta (the native form not having been seen in Glamorgan for many years). Also recorded in 2021 is a site for Viola hirta × odorata = V. × scabra (a hybrid violet) from a track side in the Vale, the first v.c. record since 1900, and only the third recent record from Wales.
In Breconshire (v.c. 42) John Crellin noted that Persicaria minor (Least Water-pepper) had only been identified and recorded once before in the county when Paul Green found it and introduced me to the species on the edge of Llangorse lake in 2013. We now know it is a species that is often impossible to find but every now and then occurs in great numbers at several waterside sites. We were lucky

that 2022 was another abundance year when Kevin McGinn wanted to collect seed for banking at NBGW and Kew. The Brecknock botany group also visited Llyn y Fan Fawr, nestling under the Black Mountain on the border with Carmarthenshire, to attempt to find rarely-recorded Isoetes lacustris (Quillwort) there, last recorded by Mike Porter in 1972. It was found only with a team effort. In as deep water as I could cope with, I could not see much at all but those on the bank were able to steer me to a ‘different plant’ (to Littorella uniflora [Shoreweed]) and there was the one and only Quillwort I could find. No doubt it is happily abundant in deeper waters.


In Monmouthshire (v.c. 35) Steph Tyler and Elsa Wood after being disappointed by how few plants they found in a survey of 92 arable fields, turned their attention to allotments. The discovery of Misopates orontium (Weasel’s-snout) growing in several plots in Chepstow allotments was the first records since 1988. They found Spirodela oligorrhiza in a water butt in a
Newport allotment; this was a vice-county first. Lemna valdiviana found in another allotment at Underwood west of Newport was a new hectad record as was Mercurialis annua (Annual Mercury) at allotments in Usk. One plant of Lepidium virginicum (Least Pepperwort) at Christchurch cemetery was our first post 2000
BSBI NEWS 152 | January 2023 67
Viola × scabra, Glamorgan. David Barden
Hymenophyllum wilsonii (Wilson’s Filmy-fern), Glamorgan. David Barden
Misopates orontium (Weasel’ssnout), Chepstow. Steph Tyler
COUNTRY ROUNDUPS: Wales
record in the vice-county. Evans in his Flora of Monmouthshire (2007) listed only five records of this casual, the last being around 1953. Colin Titcombe and Chris Hatch found about 50 plants of Impatiens noli-tangere (Touch-menot Balsam) below Piercefield car park towards the R. Wye (ST5296), the last record being from 1888 in the same place as seen in 2022. It may have originated as a garden escape from the now ruined Piercefield House.
Many new tetrad records in Monmouthshire included Erodium moschatum (Musk Stork’s-bill) in a car park at the western end of Newport, Hypericum elodes (Marsh St John’s Wort) in a pond near Newbridge, much Drosera rotundifolia (Round-leaved Sundew) in seepage areas on an old coal tip by Mynydd Varteg, Silaum silaus (Pepper Saxifrage) at Dingestow Services and Dipsacus pilosus (Small Teasel) at Llandegfedd Reservoir where Kickxia elatine (sharp-leaved Fluellen) and other plants of disturbed ground were found on the dry edges of the reservoir. It was an excellent year for Persicaria mitis (Tasteless Water-Pepper) which was fruiting in September and October on most shoals along the Wye from Monmouth down river to Llandogo.
For Anglesey (v.c. 52) the records entered so far into MapMate for 2022 include 7 new vice-county records and 32 new hectad records, a remarkably similar tally to that in 2021, but significantly less than 2020. Of the new v.c. records in 2022 two taxa are likely to be native additions. Epipactis helleborine subsp. neerlandica is a subspecies of Broad-leaved Helleborine until now known only from Kenfig dunes in S Wales. Last year Robbie Blackhall-Miles found putative material of this taxon at
Newborough in the south of the island. This year he refound the small population and took further taxonomic advice. We await genetic analysis for confirmation.
Lamiastrum galeobdolon subsp. montanum (Yellow Archangel) can also be considered native. A small population was found by Charles Aron on a steep shady lane side by a mature mixed woodland at Red Wharf Bay (SH5278). L. galeobdolon subsp. montanum appears to be absent from the mainland west of the Conwy valley and north of Porthmadog including the Lleyn (pers. comm. Wendy McCarthy). There are no historic records of Yellow Archangel on Anglesey (Roberts,1982). This present find therefore represents a significant westerly extension of its natural range in North Wales.
The other additions to the Anglesey flora are all neophytes: Cyclamen coum (Eastern Sowbread), Saxifraga × arendsii naturalised on derelict industrial ground at Amlwch, Echium pininana (Giant Viper’s-bugloss) by a footpath in Menai, Vinca major var. oxyloba (Greater Periwinkle) on a roadside near Llangristiolus and Iberis umbellata (Garden Candytuft) on a disturbed coastal roadside verge at Red Wharf Bay. The new hectad records are split between 11 native taxa and 21 introduced taxa.
This is just a small selection of the Anglesey records taken from a report by Nigel Brown and Ian Bonner, the full version of which will be in the next BSBI Wales Bulletin.
of field meetings, including several for beginners and learners organised through the hardworking Outreach Sub-committee of the Committee for Scotland. Reports of all these events will be included in the 2023 Yearbook. There was plenty of botanical survey and monitoring in addition to this, just some of which can be mentioned here.
in 2009 Jeff Waddell found four spikes of Corallorhiza trifida (Coralroot Orchid) at Heathhall Forest, the first record for Dumfriesshire (v.c. 72). In August 2022 the vice-county Botany Group revisited the location and counted 51 spikes over 1.2 hectares of wet woodland, nearly all on Sphagnum hummocks under birch. The site is very remote from the nearest known colony, at Loch of the Lowes in Selkirkshire.
There was less good news for the population of Spiranthes romanzoffiana (Irish Lady’s-tresses) found near Fort William in 2008, which seems to have disappeared due to heavy cattle trampling. However, in August James Rainey chanced upon a ‘new’

SCOTLAND
During 2022, Scotland hosted an exceptional programme
68 BSBI NEWS 152 | January 2023
Steph Tyler Joint VCR for Monmouthshire (v.c. 35)
Spiranthes romanzoffiana (Irish Lady’s-tresses), Loch Arkaig. Ian Strachan
COUNTRY ROUNDUPS: Wales / Scotland
population 15 km to the north, with 28 flowering spikes scattered along the shore of Loch Arkaig. This is only the second record in the north of Westerness (v.c. 97) for this rather erratically flowering orchid, occurring here unusually far inland.
Knoydart is one of the less-well recorded parts of Westerness. In late August Jim McIntosh and I met up with the Knoydart Foundation Rangers to explore an amazing geological feature above Inverie – the curiously named Slochd a’ Mhogha, a dramatic stream valley with deeply incised cliffs on the eastern side. This rich botanical site had not been explored for 23 years, but we refound almost all our target species and a few more besides. An outstanding feature is the abundance of Ervilia sylvatica (Wood Vetch), together with other calcicoles such as Anthyllis vulneraria (Kidney Vetch), Arabis hirsuta (Hairy Rock-cress), Silene acaulis (Moss Campion), and Dryas octopetala (Mountain Avens). New finds included Asplenium viride (Green Spleenwort), Dryopteris expansa (Northern Buckler-fern) and Hieracium holosericeum (Shaggy Hawkweed) on Sgùrr Coire Chòinnichean above.
In the same month a BSBI/ NTS ‘Rough Crew’ expedition to Torridon led by Dan Watson produced many new species for the estate, including Cherleria sedoides (Cyphel) on Liathach. There were lots of good refinds too, most notably Saxifraga rivularis (Highland Saxifrage) also on Liathach, the first record there since the 1970s. The group covered about a third of the ground in total, leaving plenty more for a future visit.
Back in Dumfriesshire, the Botany Group visited the WWT’s Powhillon Farm near Caerlaverock.
Between the sea wall and the Lochar Water, some normally wet scrapes and depressions had dried out and contained an interesting set of species including Rorippa palustris (Marsh Yellow-cress), Ranunculus baudotii (Brackish Watercrowfoot), Centaurium pulchellum (Lesser Centaury) with only three previous records in the vicecounty, Juncus ranarius (Frog Rush) with only one previous record, and Limosella aquatica (Mudwort). This last species has never been recorded in Dumfriesshire before; there is
a scatter of records to the west in Kirkcudbrightshire, but this inconspicuous annual is now a rather uncommon and declining species of muddy edges of running or standing water.

At Loch of Skene, nine miles west of Aberdeen, drainage for management purposes followed by a slow raising of the water level allowed a remarkable flora to develop on the exposed bed of the loch last summer. Most striking was a vast lawn of Eleocharis acicularis (Needle Spike-rush) which David Elston estimated to include about one billion stems!

BSBI NEWS 152 | January 2023 69
Limosella aquatica (Mudwort) (top) and Eleocharis acicularis (Needle Spike-rush) (bottom), Loch of Skene. David Elston
COUNTRY ROUNDUPS: Scotland
COUNTRY ROUNDUPS: Scotland
Several other notable species were present, including Limosella aquatica (Mudwort), as well as the locally rare Elatine hexandra (Six-stamened Waterwort). Also in South Aberdeenshire (v.c. 92) at Den of Maidencraig LNR, David and others recorded Cirsium × subspinuligerum, the hybrid between Cirsium palustre (Marsh Thistle) and C. vulgare (Spear Thistle). Identification was confirmed by Alex Prendergast and is only the second Scottish record for this surprisingly rare hybrid.
Some of the finds reported here were included in exhibits and posters at the Scottish Botanists’ Conference (SBC) which was held in November at the RBGE after two years of being online. The 140+ botanists who took part were clearly delighted to have the chance to meet up face-to-face, attend live talks and see real exhibits! The talks and presentations are all now available online on the BSBI website.
Clifton Bain gave an inspiring talk on Scotland’s rainforests and peatlands, based on his recent books on these threatened habitats. Shaila Rao of the National Trust for Scotland also gave an excellent presentation on the plant conservation work being done at Mar Lodge, in the southern Cairngorms – the largest NNR in the UK. Her talk focused on four species with small and/or isolated populations on the estate: Linnaea borealis (Twinflower), Cicerbita alpina (Alpine Blue-sow-thistle) and two montane willows, Salix lapponum (Downy Willow) and S. myrsinites (Whortle-leaved Willow). The work essentially involves planting out nursery-raised material to reinforce populations, create stepping stones and/or increase genetic diversity, but with much associated monitoring. I tend
to be rather wary of such ‘direct intervention’ projects (which seem to be on the increase), but it was reassuring that the work at Mar Lodge has some impressive research and much thought behind it. However, as Shaila said in her talk, ‘ideally we protect species before they are in need of genetic rescue’. Habitat and landscape conservation management should be the primary focus, as it is at Mar Lodge.
Brian Ballinger gave a typically thought-provoking talk on ‘Vascular epiphytes in Easter Ross’. An unexpected topic perhaps, but he has recorded a surprisingly large number of species growing on trees in his vice-county. Polypodium vulgare (Polypody) was the most common of course, but Oxalis acetosella (Wood-sorrel), Dryopteris spp. (Buckler-ferns) and Luzula sylvatica (Great Wood-rush) were
all frequent. As it happens, last spring I recorded an unwelcome epiphyte in my own vice-county –Rhododendron (super-?) ponticum (Rhododendron), growing six metres above the ground in an ancient oak at Glenuig.
There were also miniworkshops on topics such as spike-rushes, grasses and ferns, and a discussion on the impacts of Sitka Spruce (Picea sitchensis) and other invasive conifers on our flora. This lively session fed into the Committee for Scotland’s response to the Royal Society of Edinburgh’s consultation on tree planting and forestry.
Last year’s SBC marked the end of an era with the imminent retirement of Jim McIntosh. Lynne Farrell, BSBI President, led the tributes to him, highlighting his many contributions over 18 years as Scottish Officer and latterly also as Senior Country Officer. He will be greatly missed by the Scottish

70 BSBI NEWS 152 | January 2023
Jim McIntosh (right) examining Rannoch Rush with Chris Miles at Corrour, 2022. Sarah Watts
BSBI community, though he will continue to be involved as VCR for Mid Perthshire (v.c. 88) and RBGE Research Associate. But we are delighted to welcome Matt Harding, joint VCR for Stirlingshire (v.c. 86), as our new Scotland Officer, and look forward to working with him over the coming years.

 Ian Strachan
VCR for Westerness (v.c. 97)
Ian Strachan
VCR for Westerness (v.c. 97)
IRELAND
BSBI had a very active year in Ireland during 2022 with many field meetings and the Aquatic Plants Project, the latter funded by the National Parks and Wildlife Service. I led a field trip to the Back Strand at Tramore, Co. Waterford where we walked over the mudflats looking at Zostera marina (Eelgrass) and Z. noltei (Dwarf Eelgrass). These two can be surprisingly hard to identify when growing on their own. Once together they look remarkably different. We also looked at Glassworts, and updated Salicornia disarticulata (One-flowered Glasswort) for the county, as there hadn’t been any records since 2008, when the Irish branch of BSBI held their Autumn Meeting at Waterford. Even better was the update of S. emerici (Shiny Glasswort) which hadn’t been reported from the county since 2003. Not satisfied with updating the two Glassworts we found S. × marshallii a hybrid between S. disarticulata × S. ramosissima (Purple Glasswort) new for the county. Otherwise only known from Co. Wexford in Ireland. Finbarr Wallace had S. disarticulata at Ballybraher, this being the first record for Co. Cork since the publication of the Atlas of the British Flora in 1962. In the far north in Co. Down Graham Day found on Horse Island S.
emerici, a new Glasswort for the county. Graham visited Co. Cork in September and found Atriplex halimus (Shrubby Orache) near Schull on the Mizen Peninsula, a new alien for Ireland. Clare Heardman the VCR for West Cork visited Inishfarnard in June, an island in Kenmare Bay and found the largest population to date in Co Cork of Asplenium obovatum (Lanceolate Spleenwort) (photo on next page). The small fern was widespread on a dozen stone ruins on the island and was also present on nearby earth-stone walls and banks. Ironically, the name Inishfarnard – Inis Fearn Ard – translates as ‘island of the tall fern’. Earlier in the year Peter Leonard found a small population of the Lanceolate Spleenwort on Dursey Island, which was a new hectad record. Staying with ferns

BSBI NEWS 152 | January 2023 71
COUNTRY ROUNDUPS: Scotland / Ireland
Top: Paul Green and party examining Salicornia spp. (glassworts at Tramore, Co. Waterford. Clare Heardman. Bottom: Salicornia emerici (Shiny Glasswort). Graham Day
Ophioglossum vulgatum (Adder's-tongue), Inchdoney. John Deasy
Ophioglossum vulgatum (Adder’stongue) was found in three new hectads in West Cork: John Deasy found ‘plenty growing in a small area of dune slack’ at Inchdoney; Mary Sheehan found some on Crow’s Head, Beara, and Clare Heardman found it towards the western end of Inishfarnard.
Also on the coast Finbarr Wallace found a new hectad record for Limbarda crithmoides (Golden Samphire) at Ballymackeagh, Co. Cork. This is only the second record for the Vice-county of East-Cork. Shane Farrell is walking the entire coast of Ireland. On a visit to Inishturk which is about 15 km off the coast of Co. Mayo he found Crithmum maritimum (Rock Samphire), Dryopteris aemula (Hay-scented Buckler-fern), Juniperus communis subsp. nana (Dwarf Juniper) and Sagina nodosa (Knotted Pearlwort) all of which haven’t been reported since Robert Praeger visited the island back in 1906.
Shane also visited a number of other counties including Co. Longford where he found
a new native, Rosa rubiginosa (Sweet-briar) and two new aliens for the county: Petrosedum rupestre (Reflexed Stonecrop and Sarracenia purpurea (Pitcherplant). In his home county of Dublin Shane had Dactylorhiza purpurella (Northern Marshorchid) in Silogue, Ballymun, and the hybrid D. × venusta
with it and D. fuchsii (Common Spotted-orchid). The latter parent couldn’t be found anywhere. The hybrid was confirmed by Richard Bateman. His Amsinckia micrantha (Common Fiddleneck) was the second record from Rush.

There were some good finds for Killarney Fern in Ireland during 2022. In Co. Clare in January Cilian Roden and Micheline Sheehy Skeffington had this fern in the Slieve Aughty Mountains, followed in April by another site in the west of the county found by Donncha Ó Catháin. These being the first record for the sporophyte stage of the Killarney Fern in Co. Clare since 1879. Edwina Cole was leading a BSBI field meeting at Glenbower Woods, Co. Cork, in a small cave Ann Trimble found the gametophyte stage of the Killarney Fern the first record for this fern in this wood since 1844.

In Co. Clare Donncha Ó Catháin found another classic Irish plant, this time Euphorbia hyberna (Irish Spurge) by a wooded stream near Flagmount. Also new species for Co. Clare, but not native are Sisyrinchium californicum (Yelloweyed-grass) at Lough Arrow bog, found by Phoebe O’Brien, and Simon Barron and Philip Perrin had Tolmiea menziesii (Pick-a-

72 BSBI NEWS 152 | January 2023
Asplenium obovatum (Lanceolate Spleenwort), Inishfarnard. Clare Heardman
Dactylorhiza × venusta, Dublin. Shane Farrell
COUNTRY ROUNDUPS: Ireland
Euphorbia hyberna (Irish Spurge), Flagmount. Donncha Ó Catháin
back-plant) growing in damp woodland at Maryfort.

Robert and Hannah Northridge went to try and refind Gentianella amarella (Autumn Gentian) at Legalough which hasn’t been seen in Co. Cavan since 1896. But instead found one plant of Gentianella campestris (Field Gentian), a new species for the county.
From Co. Armagh John Faulkner reports one plant of Parentucellia viscosa (Yellow Bartsia) at the base of a suburban Laurel hedge in Portadown, which if not introduced, was the only new native plant to be found in

driving past the site and noticed a plant he couldn’t immediately place out of the corner of his (very sharp!) eye. John visited the site a few days later and as the Great Lettuce had ripe seed he could confirm the identity of the plant. This is the first record for Northern Ireland since 1912, and there is only one other Irish record since that date in Ireland, from Co. Cork in 2017.
The McNeill family are keeping the tradition of botany going down the generations as Ian is the joint VCR for Tyrone, and his son David is the VCR for Antrim. Ian’s grandson Samuel McNeill aged 11 found a new site for Spiranthes romanzoffiana (Irish Lady’s-tresses) by Lough Fea, Co. Tyrone.
Ciarán Flynn our new joint VCR for Co. Louth has been finding some great records for the county. His Carduus tenuiflorus (Slender Thistle) at Gyles Quay was a new hectad record. He had Antennaria dioica (Mountain Everlasting) on Slieve Foy which was new for the hectad, and only the fourth record for the county. A trip by the Rough Crew group refound Hammarbya paludosa (Bog Orchid) in the mountains where it was last reported in 1973.
of Covid-19. Seven members set out in August to record in the area, but with their sights focused on some rarer species, particularly Pyrola rotundifolia subsp. maritima (Round-leaved Wintergreen) and Hypopitys monotropa (Yellow Bird’s-nest). Luckily on this occasion both species were easy to find, which was a relief as the Yellow Bird’snest appears to have Houdini like abilities to evade recording in previous years.
 Paul Green BSBI Ireland Officer
Paul Green BSBI Ireland Officer
the county in 2022. Other new species include Nymphoides peltata (Fringed Water-lily) which was abundant on Ballylane Lough. At least 100 plants of Lactuca virosa (Great Lettuce) were found adorning the site of the former Integrated School on the Keady Road in Armagh City some of them over 2 m high. The first inkling that there might be something of interest there came from Ian McNeill, who had been
One of the alien species that is starting to turn up in many sites is Laphangium luteoalbum (Jersey Cudweed). It is now common in the southeast of Co. Wexford and has been found at a number of sites in Co. Waterford, two in Cork City and a few sites in Co. Dublin and Northern Ireland, mostly within the last five year. This year I found thousands of plants of Jersey Cudweed on waste ground in Clonmel, Co. Tipperary.
And finally, Oisín and Mairéad Duffy the VCRs for East Donegal at last were able to lead a meeting at Murvagh which had to be cancelled the two previous years because
BSBI NEWS 152 | January 2023 73
Hammarbya paludosa (Bog Orchid), Co. Louth. Ciarán Flynn
COUNTRY ROUNDUPS: Ireland
Parentucellia viscosa (Yellow Bartsia), Portadown. John Faulkner
Pyrola rotundifolia subsp. maritima (Round-leaved Wintergreen), Murvagh. Oisín Duffy
OBITUARIES
Compiled by Chris D. Preston, Obituaries Editor
19 Green’s Road, Cambridge, CB4 3EF
cdpr@ceh.ac.uk
IAN JAMES HOPKINS (1959–2022)
Ian Hopkins lived all his life in Staffordshire. He was born to parents James (known as Jim) and Sheila at Basford, Stoke-on-Trent on 5 September 1959. His interest in Staffordshire plants began in childhood and his knowledge of the botany of the county grew steadily to attain an unmatched level.
Ian attended Harpfield Primary School, Thistle Hough High School and Stoke Sixth Form College. His biology degree was gained at Staffordshire Polytechnic.
In 1982 Ian was a member of the team conducting the Staffordshire County Ecological Survey, led by Colin Hayes. This carried out a Phase I and II habitat survey, identifying key sites of nature conservation value. Colin’s abiding memory of Ian wasn’t so much his undoubted botanical and site assessment skills, but more the stoical way he accepted a lift from Colin each day from his Basford home to his place of work, throughout the seasons, in an open-top MG sports car. Colin wonders whether this was what made Ian the quiet individual he was: commuting like that wasn’t particularly conducive to chatting to get to know someone. He concludes that Ian was dedicated and committed to his work, with an attention to detail and meticulous care that were models for the whole team.

Ian became a member of the BSBI in the same year, 1983, that he began work with a group of other biologists and geologists under the leadership of Keith Bloor based at the City Museum & Art Gallery (now the Potteries Museum & Art Gallery). Within this Stoke-on-Trent Environmental Survey (which continued through 1984) his prime focus was on botanical species identification, but he also contributed to the identification of a broad range of other taxa. The City of Stoke extends 14 km north
to south and variously from 5 to 7.5 km east to west. The whole of the area was surveyed on a monad basis. Ian authored Plants, one of a series of booklets outlining the results in 1984. This extended to an impressive 65 pages and gave details of frequency, habitats and interesting notes for all the taxa that were encountered. A year later saw the publication of Ian’s Revised checklist for v.c. 39. In the course of preparing it he combined the data amassed by E.S. Edees for his 1972 Flora of Staffordshire (much of which was edited out of the original manuscript because of space restrictions applied by the publishers), the large amount of more recent information gleaned by the then very active North Staffordshire Field Club and his own observations.
Ian returned to the Polytechnic (which later became Staffordshire University) as a member of staff. He held a number of positions as a technician, the last of which was in Microbiology in the
74 BSBI NEWS 152 | January 2023
OBITUARIES
Ian Hopkins in Trentham Gardens, Staffordshire, c. 1973.
Biological Sciences Department. At one stage he was in charge of rooftop greenhouses that supplied the undergraduate identification labs. Throughout he joined field visits where his support was highly valued. There and in the labs he was the person to whom matters of detailed identification were referred. Ian was devoted to his subject and would often be found in the staff tearoom studying botanical literature. At the University he worked with Arthur Callaghan on Conidiobolos spp. and Basidiobolus ranarum, with the results being published in Fungal Ecology
Ian never drove a car, using public transport and walking considerable distances throughout the county. His holidays were spent botanising elsewhere in the British Isles. All his observations were recorded systematically in notebooks.
A small committee was set up in 1998 to prepare a new Flora for Staffordshire and he was deeply involved from the start. At the end of each season he meticulously copied his data on to record cards for each tetrad. He was one of the two most prolific annual recorders. The Flora was published in 2011, with Ian as joint author. Although he had little to say in face-to-face conversations, and even less on the telephone, all his written communications were neatly and clearly presented and packed with relevant and interesting details. He had an exceptional ability and knowledge that enabled him to identify any unusual garden throw-out. Of the
OBITUARY NOTES
Since we compiled the last Obituary Notes, news has reached us of the death of the following members or former members. We send our sympathy to their families and friends.
Mr D. Albon of Bury St Edmunds, a member for 5 years; Mr G.D. Field of New Milton, a member for 54 years; Mrs S. Gilmour of Sheffield, a member for 71 years; Mr E.F. Greenwood of Wirral, an Honorary Member and a member for 59 years; Mr P.C. Heathcote of Longfield, a member for 23 years; Prof. V.H. Heywood, former member and editor of Flora Europaea ; Dr J.D. Hopton
various chapters that he wrote for the Flora, the one detailing the history of botany in the county was particularly masterly.
Ian became Joint Vice-County Recorder for v.c. 39 in 2014. He continued detailed recording after 2011 until the beginning of the Covid epidemic, totalling many tens of thousands of further new records for that decade. With Covid, he made the decision to move the five miles from Basford to Trentham in order to help support his elderly father who was not in good health. Tragically, after a short time he also became ill and was diagnosed with colon cancer. In spite of treatment this led to his early death eighteen months later.
I am grateful to Ian’s sister Julie and to Dave Skingsley, Colin Hayes and Keith Bloor for sharing their memories of Ian.
References
Hopkins, I.J. 1984. The Wildlife of Stoke-on-Trent. 5. Plants. City Museums and Art Gallery, Stoke-on-Trent.
Hopkins, I.J. 1985. Staffordshire Plants and Ferns, a Revised Checklist City Museums and Art Gallery, Stoke-on-Trent.
Hopkins, I.J. & Callaghan, A.A. 2010. Survival of Conidiobolos spp. and Basidiobolus ranarum in relation to relative humidity and temperature. Fungal Ecology 3(3): 148–159.
Hawksford, J.E. & Hopkins, I.J. 2011. The Flora of Staffordshire Staffordshire Wildlife Trust.
John Hawksford
of Harrogate, a member for 50 years; Ms E.J. McDonnell of Wedmore, a member for 44 years and joint VCR for N. Somerset; Miss J. Mossop of Egremont, a member for 41 years; Prof. C.D. Pigott of Grange-over-Sands, a member for 77 years and referee for Thymus and Tilia; Mr S.M. Povey of Petersfield, a member for 44 years.
Chris D. Preston, Obituaries Editor
Assisted by the Membership Secretary, Gwynn Ellis. Date of compilation 1 December 2022.
BSBI NEWS 152 | January 2023 75
OBITUARIES
REVIEWS
Compiled by Clive Stace, Book Reviews Editor Appletree House, Larters Lane, Middlewood Green, Stowmarket, IP14 5HB cstace@btinternet.com
Where the Wild Flowers Grow

Leif
Bersweden
Hodder & Stoughton, London, 2022; pp. ix + 390, with 16 pp. of coloured photographs; hbk £20.00.
ISBN 9781529349535
Recent publicity concerning the harmful effects of climate change has led to a renewed awareness of the value of our wildlife, and of the losses already sustained in natural habitats. A high proportion of the population now lives in an urban environment and has little incentive to visit the countryside, with the result that there is a general lack of knowledge of the natural environment.
Leif Bersweden developed an interest in plants at an early age, with a special love for his local Wiltshire flora. He would botanise at every opportunity, puzzling out the names of the flowers he found and welcoming the reappearance of old favourites through the seasons. He may be known to many BSBI members as the author of The Orchid
Hunter, an account of his journey around Britain to find all of our native orchid species within the space of one year. Later, his studies completed, he resolved to visit all the special wildflower habitats in Britain and Ireland, to record what was there before it is too late. This book is an account of these further journeys, in more or less chronological order. They include many wellknown botanical hotspots such as chalk grassland, Ben Lawers, the Broads, and – my favourite – West Cork. The accounts are part narrative, and part description of habitats and plants, interspersed with many interesting nuggets of information, such as an explanation of the optical properties of buttercup petals.
It is a drawback that he uses only English plant names in the text, even for bryophytes, on the grounds that this makes the book more accessible. He forestalls criticism by providing the source used for the English names, and advising readers to visit the book’s dedicated website which has photographs of all the plants mentioned in the text, with their Latin names. Since it is quite a hefty tome, it should surely have been possible to provide a list somewhere in the book. However, he writes with such infectious enthusiasm about the glories of our flora, and the need to preserve it, that it would be churlish to carp.
At the end there is much good advice about how to get started with plant hunting: which books to get, social media to follow and
useful societies to join (the BSBI gets a special mention as ‘the first organisation to become familiar with’).
Overall, the book is an engaging and enjoyable read. It would make a good present for beginners and plant lovers who would like to increase their knowledge, and more experienced botanists could enjoy accounts of places and plants they have seen in the past.
Mary Clare Sheahan
Great Trees of Great Britain and Ireland

Tony Hall
Royal Botanic Gardens, Kew, 2022; pp. 184, with many coloured photographs; hbk £25.00.
ISBN 9781842467466
Famously, there is no universally agreed definition of a tree. Indeed, Lord Denning in a landmark ruling stated that ‘anything that one would ordinarily call a tree is a tree’.
Great Trees of Britain and Ireland clearly falls into the Denning camp. The author declines to
76 BSBI NEWS 152 | January 2023
REVIEWS
define what he considers a tree but a quick flick through the many high quality photographic portraits will leave the reader in no doubts: these are definitely trees and magnificent ones at that. On first impressions, this is an attractive, informative publication, suitable for any coffee table, but is it worthy of the botanist’s bookshelf?
The aim of this book is to illustrate and catalogue a publicly accessible selection of the grandest, oldest, largest and most notable trees of the British Isles, the ‘essential companion to exploring these natural beauties, telling their stories and helping to protect them’. We are told that Britain and Ireland boast the most ancient oaks and yews in Europe and have a wealth of forests that can be traced back centuries, with trees that are hundreds of years old, some truly majestic. Nothing to disagree with there.
The rather lengthy introduction covers British and Irish woodland history, traditional management practices and the importance of trees for biodiversity. A particularly interesting section explains the role of mycorrhiza and what is neatly referred to as the Wood Wide Web. The introduction concludes with a section on pollarding but then, quite abruptly, jumps straight into a list and a map of the trees that are subsequently profiled.
Somewhat surprisingly, the first few of the trees listed are non-native species, as are at least a third of the total, which is rather incongruous given that there has been little or no previous mention of this, all discussion having so far been mainly concerning native trees and woodland. The tree profiles themselves are interesting and informative, but it is not entirely made clear that many are introduced species. Personally, I
have no problem with non-native trees, in their place, but the lack of explanation or reference to the long and glorious British and Irish tradition of plant hunting and collecting of exotic plants from far and wide across the globe is in my view a missed opportunity, as well as being potentially misleading to the reader.
Other, relatively minor issues are the inconsistent use of scientific names after common names and several typos, though these are not excessive and do not detract from the narrative. Whilst most of the photos are high quality, many are printed across pages resulting in ugly folds down their centres, which for me detracts somewhat from the beauty and magnificence they are attempting to portray.
Despite this, I enjoyed the book and hope that other readers will be inspired to visit these trees and see them in context. There are some amazing photographs of amazing trees here, many I already know, but many I don’t and am now inspired to visit myself.
Cameron S. Crook
In 1731 Edward Lhwyd was famously called ‘The best Naturalist now in Europe’ by Sir Hans Sloane, and he has been variously described as botanist, archaeologist, geologist, linguist, chemist, palaeontologist, topographer and antiquary – a wide range of interests not unusual in the 17th century. John Ray thought that he was … ‘most expert not only in botany but in all of Natural History’. Who was Edward Lhwyd?

Edward Lhwyd was born in 1660 and brought up at Llanforda, Oswestry. His father, Edward Lloyd, was a minor landowner who had fallen on bad times during the English Civil War, and his mother was Bridget Pryse, a member of the influential Gogerddan family of north Cardiganshire. His parents never married. At Llanforda he developed an interest in science, especially botany – his father was a keen gardener and horticulturalist – and from an early age he enjoyed walking the hills collecting plants, or as his father famously put it ‘Neddy’s gone a-simpling’.
ISBN 9781786837820
Young Edward Lhwyd was greatly influenced by Edward Morgan, the gardener and botanist at Llanforda. Morgan, who had trained in London and was well known as a skilful plantsman and as a Welsh speaker, acted as interpreter for a group of English botanists, led by Thomas Johnson, who had made the first scientific ascent of Snowdon in 1636. At the age of 22 Edward Lhwyd went up to Jesus College, Oxford, where he found frequenting Oxford’s Physic Garden far more congenial than studying law. Meanwhile, he had sent Ray a list of the flora of Llanberis, which had greatly impressed Ray. Lhwyd never lost his love of botany and became the acknowledged expert on the
BSBI NEWS 152 | January 2023 77
Edward Lhwyd
Brynley F. Roberts University of Wales Press, Cardiff, 2022; pp. xxviii + 321, with 9 B & W figures: pbk £16.99.
REVIEWS
mountain plants of North Wales. His most notable discovery was the Snowdon Lily, named after him – Lloydia serotina, now renamed Gagea serotina.
At Oxford, Lhwyd soon came to the attention of the authorities, and was appointed assistant keeper of the Ashmolean Museum where he became especially interested in the large shell collection which he catalogued meticulously. His interests widened and later, in 1691, he was appointed Keeper of the Ashmolean. His studies bore fruit when he published his Ichnographia – the first systematic catalogue of British fossils. He was invited to write the entries on the Welsh counties in the new edition of Camden’s Britannia, which in turn led to his major project – a Grand Tour of Wales, Scotland, Ireland, Cornwall and Brittany, studying nature, customs, and especially languages. Of the proposed publications, only one volume appeared, dealing with the relationships of the Celtic languages. Sadly, Lhwyd died at the age of 49, but not before establishing himself as a leading exponent of botany, palaeology, archaeology and linguistics.
This ‘life’ of Lhwyd, in the series Scientists of Wales, is written by Dr Brynley Roberts, former Professor of Welsh at Swansea University and Librarian of the National Library of Wales. His meticulous research and enthusiasm for his subject are clearly evident; in his own words ‘Edward Lhwyd was my companion for many years’. This is both a scholarly and readable book and is clearly the standard account of a remarkable life.
Goronwy Wynne
Guide Expert des Carex de France.

D. Hamon, with drawings by D. Mansion, J.L. Castillo & R. Portal Éditions Biotope, Mèze, 2022; pp. 384, with numerous photographs, line-drawings and maps; sbk 39 Euros. ISBN 9782366622928
The happy few among us British and Irish botanists who have one or more of Robert Portal’s various books on grass genera in France will be interested to know of this new, somewhat similar, book on Carex, containing many of Portal’s excellent drawings among equally fine ones by two other artists. The author is David Hamon, and the book is described as a ‘field identification manual’, although it is about the same size and 80 pages thicker even than John Richards’s Field Handbook of our dandelions. Even for those unfamiliar with French, the text should be reasonably easy to follow. The keys, very well-illustrated with drawings and photos, work well. In the main text, the species are in alphabetical order. Each has a two-page spread with a good description, accounts of distribution and ecology, a distribution map showing occurrence by départements, diagrams of seasonality and altitude, two photos, one of habit and the other of
inflorescence, and a whole page of exceptionally fine drawings of habit, inflorescence, glume (male and female where appropriate), utricle, nut, ligule, etc. A total of 120 species is covered, and as only six British and Irish species are not included (Carex aquatilis, C. bigelowii, C. norvegica, C. rariflora, C. salina and C. saxatilis) the book is applicable to most of our area.
The taxonomy and nomenclature of the natives are very similar to those in Stace’s New Flora ed. 4, except that C. leersii is accepted as a species, C. filiformis is called C. lasiocarpa and C. simpliciuscula is called C bipartita; C. viridula is called C. oederi and includes subsp. pulchella (subsp. bergrothii is not included). Surprisingly, only two aliens are given, C. crawfordii and C. vulpinoidea, but Tison & Foucault’s Flora Gallica gives no more either. A disappointment is that although 127 hybrids are listed, and it is explained how hybrids in general may be recognised, none of them is described or keyed, or details of their occurrence indicated.
(Our Handbook by Jermy et al., Sedges of the British Isles (2007) is cited though as a helpful source.) Three other hybrids, C × microstyla, C × turfosa and C. × xanthocarpa, described as rare but distinctive, are however briefly described and keyed.
The introductory chapters are very informative and helpful and, as everything else, handsomely illustrated, the one on morphology especially. The habitats and ecology chapter gives lists of species for each of the main habitats. Following the main keys is a chapter containing another, more complex but useful and ingenious version of the keys, the species grouped by similar-looking inflorescences,
78 BSBI NEWS 152 | January 2023
REVIEWS
and with tables and photos as well as numerous short keys. At the end of the book are tables showing phenology and altitudinal distribution, a glossary and a bibliography. Errors seem negligible, though the description and drawing of the ligule of C. binervis give it as much too long and quite the wrong shape.
A paperback with sewn binding, it opens well and seems very robust. The size and weight of the book and the complexity of the admittedly extremely good keys would make it awkward to use in the field, but from every other point of view Carex de France is very strongly recommended. Anyone interested in the
LETTERS
HYPOPITYS MONOTROPA ON BROWNFIELD SITES IN SOUTH-EAST WALES
Iwasinterested to read the article by Richard Milne ( BSBI News 151: 9–12) on Hypopitys monotropa (Yellow Bird’s-nest) growing in Scotland on brownfield sites and the discussion of how this species spreads.
In Monmouthshire (v.c. 35) we only have three, possibly four known locations for the species. One site has typical habitat, the species being under Fagus sylvatica (Beech) on limestone at the Blackcliff in the lower Wye Valley (ST59J). The number of flowering spikes varies between years but the highest count is 63. Within the county but just outside the vicecounty in Breconshire (v.c. 42), is another population in similar habitat in Cwm Cydach Gorge. The other two of our sites for the species are on derelict industrial or brownfield sites.
On 9 July 2009 Heather Colls found a colony of about 100 spikes of Hypopitys monotropa subsp. hypophegea at Uskmouth, near Newport at the northern edge of what is now the Newport Wetlands Reserve (ST3383). Much of the reserve was created on ash waste from Uskmouth Power Station. The plants found by Heather were growing on ash in
genus, or intending to become interested, should acquire it. Elements in its format could usefully be adopted in our own Handbooks, Dandelions being perhaps our nearest approach to it in style.
Arthur O. Chater
willow Salix sp. and other scrub. The population has been reported on several occasions since. This site is more than 20 miles from the Blackcliff site.
Coincidentally, on 10 July 2009 I was botanising with Trevor Evans at the former Alpha Steel works near Newport, just north of Uskmouth in ST3384. We were checking numbers of Epipactis palustris (Marsh Helleborine) around an old settling lagoon and nearby on industrial waste I found patches of Hypopitys monotropa subsp. hypophegea and close by, also under small willows, were patches of Pyrola rotundifolia (Round-leaved Wintergreen) growing around the old ponds. (All of our seven sites for P. rotundifolia in v.c. 35 are on former coal tips or other brownfield sites.) There is also a record from Lucy Kelly sent to the South East Wales Biological Record Centre (SEWBReC) of Hypopitys monotropa at Malpas, north of Newport (ST3190) in July 2012 but this was not confirmed and no photograph or details of the habitat and substrate were provided.
It appears that in south-east Wales to find Hypopitys monotropa or Pyrola rotundifolia you need to botanise on brownfield sites.
Stephanie J. Tyler Joint Vice-county Recorder for Monmouthshire (v.c. 35)
BSBI NEWS 152 | January 2023 79 REVIEWS / LETTERS
2022 BSBI Photographic Competition winning entries (see p. 60)
Winter: Heracleum sphondylium (Hogweed), Huby, near York. Alwyn Craven (top)

Spring: Oxalis acetosella (Wood-sorrel), Surrey. Gillian Elsom (bottom)

80 BSBI NEWS 152 | January 2023
2022 BSBI Photographic Competition winning entries (cont.)
Summer: Ophrys insectifera (Fly Orchid), Surrey. Gillian Elsom (left)

Autumn: Spiranthes spiralis (Autumn Lady’s-tresses), Hampshire. Gillian Elsom (right)

BSBI President Micheline Sheehy Skeffington with Chris Miles (Chair of the Trustees) and Steve Gater (Hon. General Secretary) at the British & Irish Botanical Conference, November 2022. Julia Hanmer

BSBI NEWS 152 | January 2023 81
BSBI PHOTOGRAPHIC COMPETITION 2022 / BRITISH & IRISH BOTANICAL CONFERENCE









 Lynne Farrell, Member
Lynne Farrell, Member




 ERIC GREENWOOD
ERIC GREENWOOD








































 Mike Crewe mikedcrewe@gmail.com
Mike Crewe mikedcrewe@gmail.com



























 RODNEY BURTON
RODNEY BURTON


 HOWARD M. BECK
HOWARD M. BECK






















 Ian Strachan
VCR for Westerness (v.c. 97)
Ian Strachan
VCR for Westerness (v.c. 97)






 Paul Green BSBI Ireland Officer
Paul Green BSBI Ireland Officer

















